Highly expressed Staphyloccocus aureus alpha-acetolacetate decearboxylase by utilization of recombinant escherichia coli
A technology of acetolactate decarboxylase and Escherichia coli is applied in the field of genetic engineering and enzyme engineering to improve production efficiency and shorten the aging cycle of beer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: Construction and transformation of recombinant plasmid pET-28a-saald
[0022] [1] Extract chromosomal DNA from Staphylococcus aureus as a template, the extraction method is as follows: use an inoculation loop to pick a single colony from a fresh Staphylococcus aureus plate and suspend it in 0.5mL sterilized double distilled water, boil it for 5 -10min, centrifuge at 8000r / min for 10min, take the supernatant into a clean 1.5mL centrifuge tube, and store in a -20°C refrigerator for later use.
[0023] [2] Using the total DNA of Staphylococcus aureus as a template, use the primers provided in Example 1 for PCR amplification. The amplification conditions are: 94°C pre-denaturation, 5min, one cycle; 94°C denaturation, 1min, 56°C annealing, 1min, 72°C extension, 1min30s, 30 cycles; 72°C, 10min, one cycle; 15°C, 10min, one cycle. PCR amplification system: template (Staphylococcus aureus chromosomal DNA) 2 μL, upstream and downstream primers 0.5 μL each, dNTP Mix...
Embodiment 2
[0026] Example 2: Expression of α-acetolactate decarboxylase and assay of enzyme activity
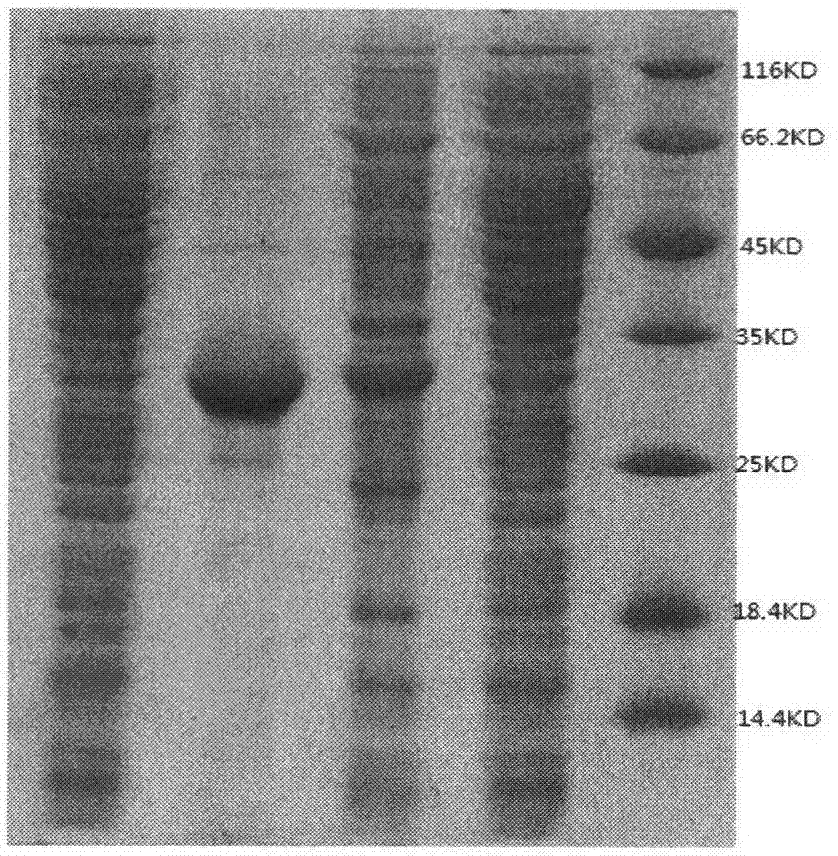
[0027] [1] Inoculate the strains stored in cryopreserved tubes in Example 1[4] into 10 mL of liquid LB medium, cultivate overnight at 37°C with shaking, transfer at 1% inoculum size the next day, and cultivate to OD at 37°C 600Add IPTG at a final concentration of 1 mmol / L at about 0.6 to 0.8, and place on a shaker at 30°C overnight to induce expression. Take 1mL of the obtained bacterial solution in a 1.5mL centrifuge tube, centrifuge at 8000r / min for 2min, pour off the supernatant, add 100L Tris-HCl buffer solution with pH 6.8 to mix the bacteria, add 30L protein gel loading buffer, boil water Bath for 30 minutes to denature the protein, centrifuge at 8000r / min for 2 minutes, take the supernatant for SDS-PAGE detection, wherein SDS-PAGE uses 5% separating gel and 15% stacking gel, adopts discontinuous vertical electrophoresis, and uses Coomassie brilliant blue R250 For staining, E.coi...
Embodiment 3
[0029] Example 3: Purification and Enzymatic Properties of α-Acetolactate Decarboxylase
[0030] [1] Pure ALDC was obtained after the crude enzyme solution was purified by Ni-NTA column. The specific activity of the purified enzyme solution was 469.85U / mg, the purification factor was 8.36 times, and the recovery rate was 87.65%.
[0031] [2] Optimum pH and pH stability: Prepare substrate solutions with different pH (2-12) with substrate buffer MES II, dilute the enzyme solution with MES I to a certain number of times, and then mix 380 μL of substrates with different pH with 20 μL of the diluted enzyme solution was reacted, and the enzyme activity under different pH conditions was measured and compared to determine the optimum pH value of the enzyme reaction. Prepare enzyme activity assay buffer MES I with different pH (5-10), and use them to dilute the enzyme solution to obtain enzyme solution with different pH. Let the enzyme be in different pH environments, take samples at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com