Porphyrin copolymer containing thienothiadiazole units, preparation method and uses thereof
A technology of porphyrin copolymer and thiadiazole, which can be used in chemical instruments and methods, electrical components, and final product manufacturing, etc., and can solve problems such as low photoelectric conversion efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
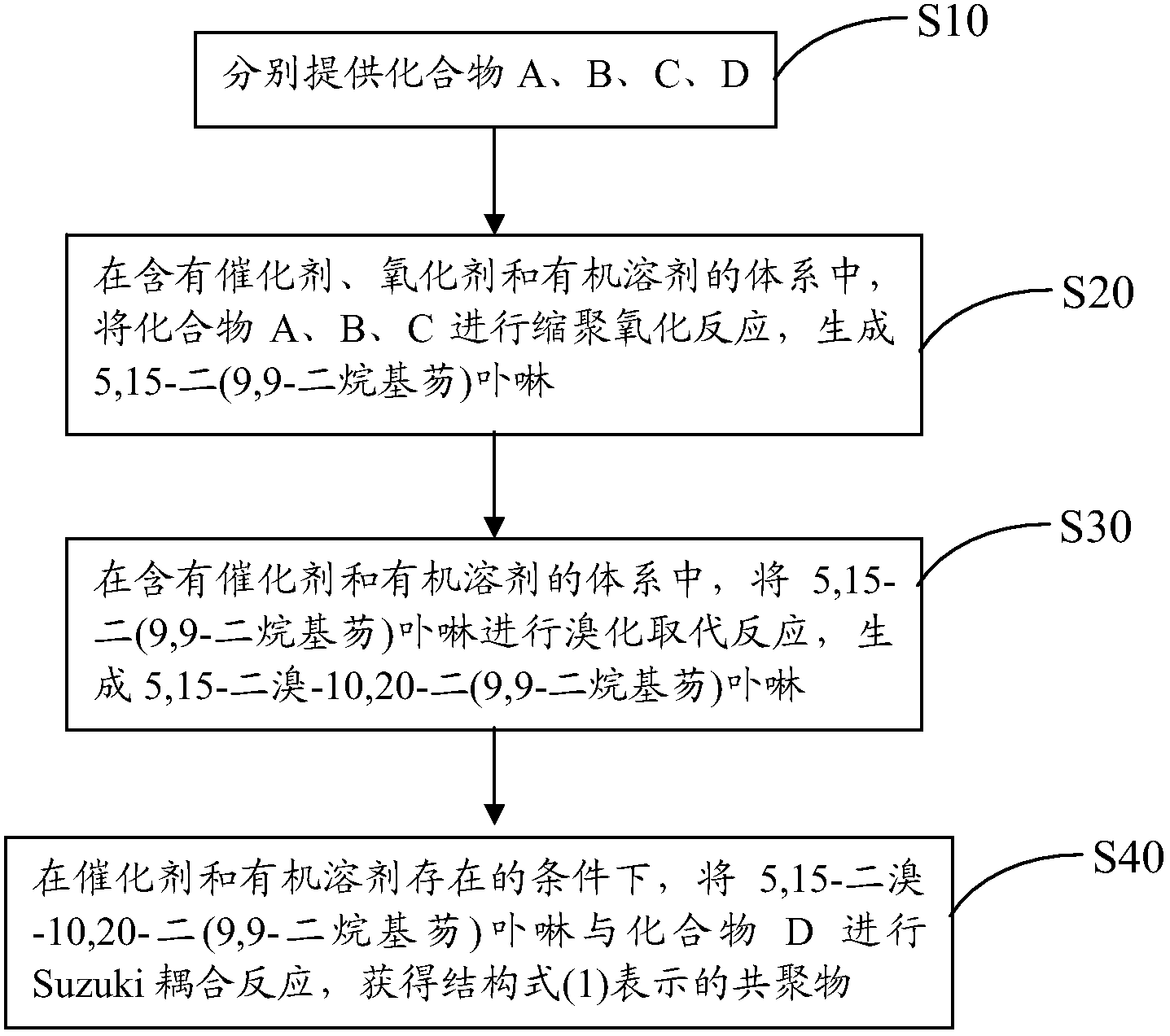
[0033] see figure 2 , the preparation method of above-mentioned porphyrin copolymer containing thienothiadiazole unit comprises the steps:
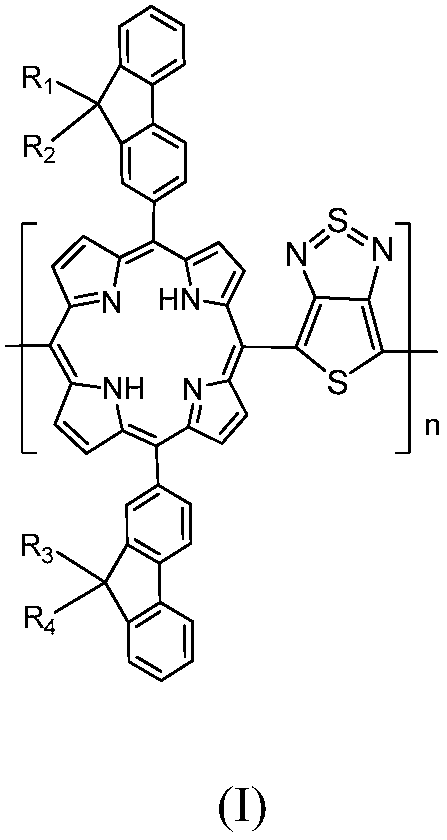
[0034] S10, respectively provide compounds A, B, C, D represented by the following structural formula,
[0035] where R 1 , R 2 , R 3 , R 4 for the same or different C 1 -C 32 the alkyl group;
[0036] S20, in a system containing a catalyst, an oxidizing agent and an organic solvent, the compounds A, B, and C are subjected to polycondensation and oxidation reactions to generate 5,15-di(9,9-dialkylfluorene)porphyrin;
[0037] S30, in a system containing a catalyst and an organic solvent, 5,15-bis(9,9-dialkylfluorene)porphyrin is subjected to a bromination substitution reaction to generate 5,15-dibromo-10,20-bis( 9,9-dialkylfluorene) porphyrin;
[0038] S40, in the presence of a catalyst and an organic solvent, 5,15-dibromo-10,20-two (9,9-dialkylfluorene) porphyrin and compound D are subjected to a Suzuki coupling reaction to ob...
Embodiment 1
[0079] The present embodiment 1 provides 10,20-bis(9,9-dioctylfluorene)porphyrin-thieno[3,4-c][1,2,5]thiadiazole copolymer with the following structure. The structural formula of the compound is shown in the product of the reaction formula of the fourth step. The preparation steps of above-mentioned polymer are as follows:
[0080] 1. 5,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) thieno[3,4-c][1,2,5] The preparation of thiadiazole, its preparation involves reaction formula as follows:
[0081]
[0082] The specific process is: under the protection of nitrogen, add p-9,10-dibromothieno[3,4-c][1,2,5]thiadiazole (9.0g, 0.03mol) into the three-necked flask, add 200ml of tetrahydrofuran solvent, slowly inject n-butyllithium (25.2mL, 2.5M, 0.06mol) with a syringe at -78°C, continue stirring for 2h, inject 2-isopropyl lithium with a syringe at -78°C Oxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (13 mL, 0.06 mol), stirred overnight at room temperature. Saturated aqueous sodium...
Embodiment 2
[0094]This Example 2 provides 10-(9-methyl-9-octylfluorene)-20-(9-decyl-9-hexadecylfluorene)porphyrin-thieno[3,4-c with the following structure ][1,2,5]thiadiazole copolymer, the structural formula of this polymer is shown in the product of the reaction formula of the fourth step. The preparation steps of above-mentioned polymer are as follows:
[0095] 1. 5,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) thieno[3,4-c][1,2,5] The preparation of thiadiazole is detailed in Example 1.
[0096] Two, the preparation of 10-(9-methyl-9-octylfluorene)-20-(9-decyl-9-hexadecylfluorene) porphyrin, this step involves the reaction formula as follows:
[0097]
[0098] The specific process is: set up an anhydrous and oxygen-free device, weigh the intermediates 9-methyl-9-octylfluorene (0.32g, 1mmol), 9-decyl-9-hexadecylfluorene (0.56g, 1mmol) ), dipyrromethane (0.30g, 2mmol), dissolved in 250ml of dichloromethane, nitrogen 30min, inject 2ml of trifluoroacetic acid into the syringe, sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com