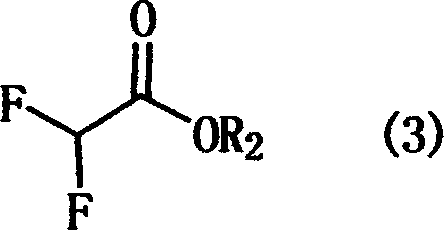
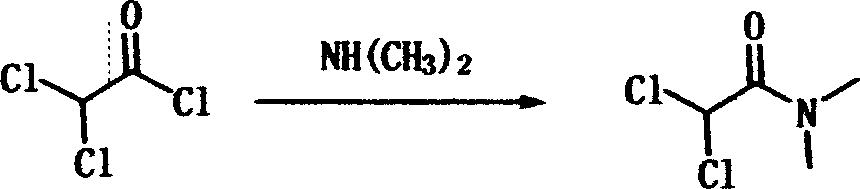Industrialized synthetic method of ethyl difuoroacetate
A technology of difluoroacetate and synthesis method, which is applied in chemical instruments and methods, preparation of carboxylic acid esters, preparation of organic compounds, etc., can solve the problem of inability to synthesize products, diethylamine is not suitable for large-scale production, and does not have issues of authenticity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0027] 1.1 Add 2Kg of toluene and 500g of chloroacetyl chloride into a 5L four-necked flask, seal the system (with a buffer ball), cool down in an ice-salt bath to 0-5°C, and slowly and quantitatively inject 315g of dimethylamine gas into the dimethylamine cylinder. Naturally rise to room temperature and react for 3-5 hours, filter with suction, wash the filter cake with an appropriate amount of toluene, concentrate the filtrate to obtain the crude product, and rectify at 57-62°C to obtain the pure product N,N-dimethyl-dichloro Acetamide 499.7g, yield 94.5%, collect amine salt filter cake for recovery of dimethylamine for later use.
[0028] 1.2 Add 468g of pure N,N-dimethyl-dichloroacetamide, 1170g of diethylene glycol, and 442g of anhydrous KF to a 3L four-neck flask, connect to a drying tube, stir, and heat up to 150-160°C for 12-16 hours . After cooling down to room temperature, rectification under reduced pressure at 65-68°C (vacuum degree 70-80 mmHg) yielded 283.6 g of ...
example 2
[0031] 2.1 Add 2Kg of toluene and 500g of dichloroacetyl chloride into a 5L four-neck flask, seal the system (with a buffer ball), cool down in an ice-salt bath to 0-5°C, slowly and quantitatively inject 315g of dimethylamine gas into the dimethylamine cylinder, and end Then naturally rise to room temperature to react for 3-5 hours, filter with suction, wash the filter cake with an appropriate amount of toluene, concentrate the filtrate to obtain the crude product, (vacuum degree 0-5mmHg) rectify at 57-62°C to obtain the pure product N,N-dimethyl-di Chloroacetamide 506.6g, yield 95.8%, collect the amine salt filter cake for recovery of dimethylamine for later use.
[0032] 2.2 Add 468g of pure N,N-dimethyl-dichloroacetamide, 1170g of diethylene glycol, and 442g of anhydrous KF to a 3L four-neck flask, connect to a drying tube, stir, and heat up to 150-160°C for 12-16 hours . After cooling down to room temperature, rectification under reduced pressure at 65-68°C (vacuum degree...
example 3
[0035] 3.1 Put the dimethylamine hydrochloride collected by amination into the flask after the esterification reaction, add 500g of toluene, add 40% concentrated NaOH aqueous solution dropwise, control the material at 40-50°C, and the dimethylamine gas generated by the reaction passes through After drying the tube with alkali, it is directly passed into a 5L amination (500g dichloroacetyl chloride) reaction flask. After the dimethylamine gas is released, the amination reaction takes 1 hour, and the reaction is quenched with ethanol for sampling and filtration. 6-7% is detected by GC. The raw material dichloroacetyl chloride was not completely reacted, and 22g of dimethylamine gas was introduced into the dimethylamine steel cylinder, then raised to room temperature and reacted for 3 to 5 hours, then suction filtered, the filter cake was washed with an appropriate amount of toluene, and the filtrate was concentrated to obtain a crude product, (vacuum degree 0 to 5mmHg ) at 57-62°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com