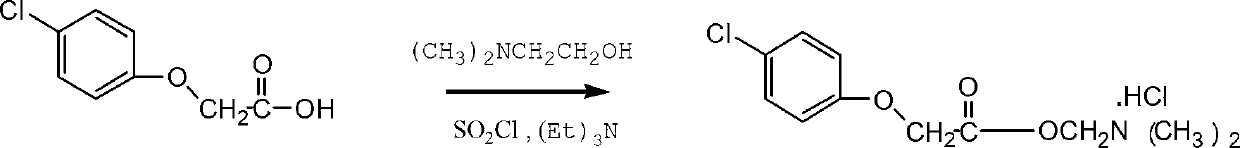Meclofenoxatum preparation method
A technology for meclofenxetil hydrochloride and quaternary ammonium salt, which is applied in the preparation of organic compounds, chemical instruments and methods, preparation of amino hydroxy compounds, etc., can solve the problems of impurities exceeding the standard, unsuitable for control, and difficult to remove, and improves the yield. , avoid residue and decomposition, improve the effect of utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
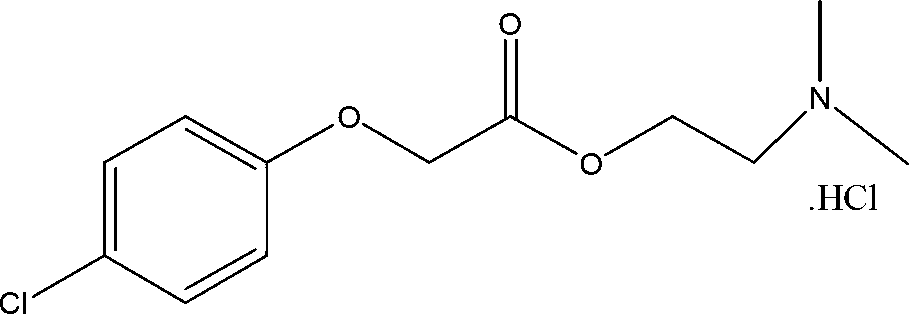
[0028] Put 230g (1.23mol) of p-chlorophenoxyacetic acid and 23g (0.23mol) of triethylamine into 600ml of toluene in a three-necked 1L reaction flask, add 163g (1.37mol) of thionyl chloride under stirring at room temperature, and then raise the temperature to 70°C Keep warm for 30 minutes, continue to heat up to 100°C, keep the temperature between 70~100°C, add 113g (1.27mol) of dimethylaminoethanol, keep warm for 2 hours after the dropwise addition, recover the solvent until dry, add 250g of isopropanol to heat Dissolve, add appropriate amount of activated carbon to decolorize, filter, cool and stir to precipitate 289.5g of white crystals, yield: 82.5%, melting point 138~141°C is the product meclofenoxate hydrochloride. The structure was confirmed by infrared spectroscopy (IR (KBr) δ: 2940, 1740, 1580, 1490, 1380, 1090, 810, 710cm-1)
[0029] The reaction equation is as follows:
[0030]
[0031] 4-chlorophenoxyacetic acid, dimethylaminoethanol Meclofenoxate hydrochloride ...
Embodiment 2
[0033] Put 230g (1.23mol) of p-chlorophenoxyacetic acid and 31.8g (0.25mol) of N,N-diisopropylethylamine into 600ml of toluene, add 163g (1.37mol) of thionyl chloride under stirring at room temperature, and then raise the temperature to 75 Keep warm at ℃ for 30 minutes, continue to heat up to 100℃, keep the temperature between 70~100℃, add 113g (1.27mol) of dimethylaminoethanol, keep warm for 2 hours after the dropwise addition, recover the solvent until dry, add 250g of absolute ethanol Heat to dissolve, add appropriate amount of activated carbon to decolorize, filter, cool and stir to precipitate 290.1g of white crystals, yield: 82.3%, melting point 137~140℃.
Embodiment 3~ Embodiment 14
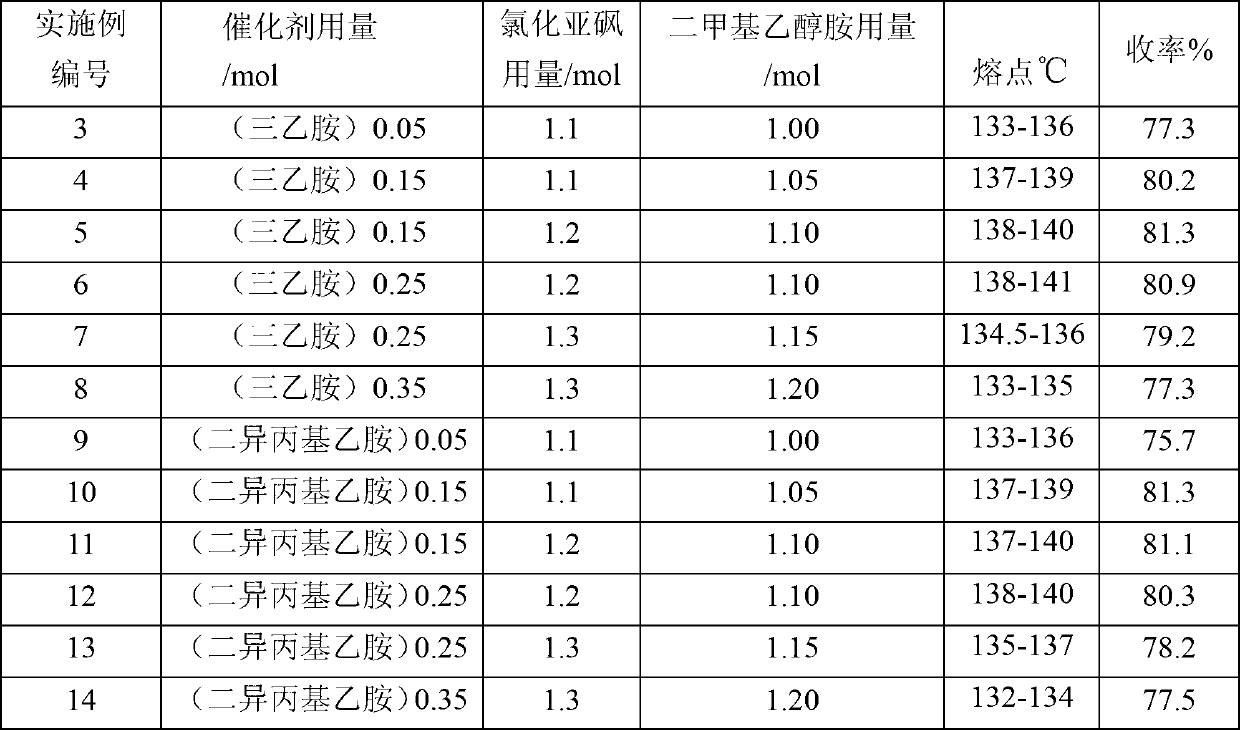
[0035] Except that the process parameters of embodiment 3~embodiment 14 are listed in table 1, all the other are with embodiment 1.
[0036] Process parameter, product purity and yield of table 1 embodiment 3~embodiment 12
[0037]
[0038] Note: The amount of each component above is relative to the molar amount of p-chlorophenoxyacetic acid
[0039] As can be seen from Table 1, the process parameters adopted in Examples 4, 5, 6, 10, 11 and 12 fall within the scope according to the present invention, that is: p-chlorophenoxyacetic acid, nitrogen-containing organic base, chlorinated The molar ratio of sulfone and dimethylethanolamine is 1:0.15~0.25:1.1~1.25:1.00~1.10 These examples have achieved good yields, the product yields are all above 80%, and the melting point is 137~141°C. Meet the requirements of Chinese Pharmacopoeia 2010 edition.
[0040] And embodiment 3,7,8,9,13,14 are inferior to embodiment 4,5,6,10,11 and 12 aspect melting point and yield because process par...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com