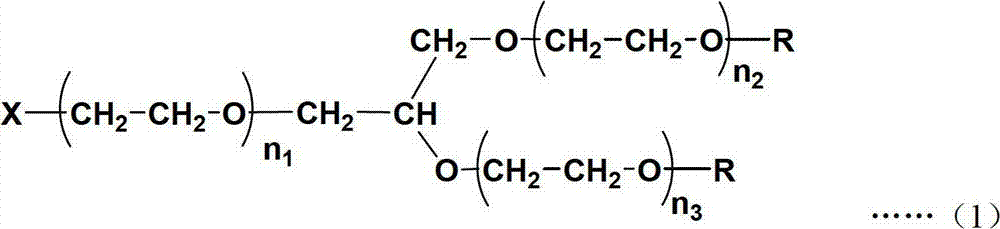
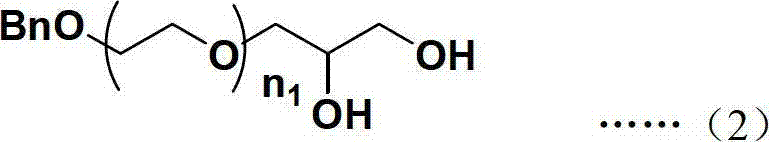
Single active functional group-containing Y-type polyethylene glycol and preparation method thereof
A technology of active functional groups and polyethylene glycol, which is applied in the preparation of carriers for biomedical preparations, Y-type polyethylene glycol and its preparation, can solve the problems of inability to perform specific reactions, instability, and low drug coupling efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
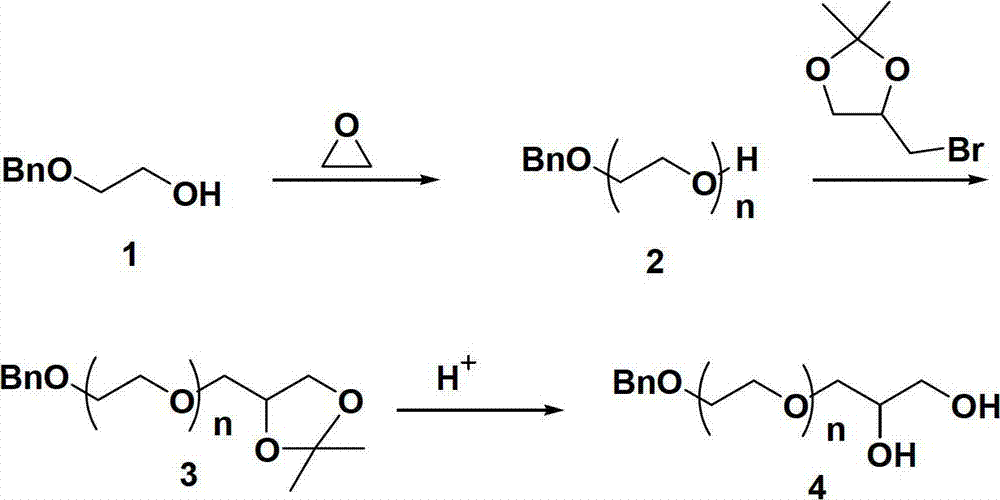
[0039] Preparation Example 1: Preparation of Compound 1
[0040] Under a nitrogen atmosphere, potassium hydride (200 mg, 5.0 mmol), tetrahydrofuran solution (40 mL), and 2-benzyloxyethanol (0.76 g, 5 mmol) were successively added to a 100 mL dry and clean double-necked round-bottom flask. Stir for four hours. Subsequently, ethylene oxide (30 g, 682 mmol) was added through a constant pressure dropping funnel in an anhydrous and oxygen-free system and reacted at room temperature for 24 hours. Then add excess bromoacetone glycalol to capping active oxygen anion, continue to react at 50 DEG C for 24 hours. The generated salt was separated by centrifugation, and then the solvent of the supernatant was evaporated by rotary evaporation, and precipitated in diethyl ether, and the precipitate was dried in a vacuum oven at 45° C. for 12 hours to obtain compound 1. Compound 1 1 H NMR is: 1 H NMR (CDCl 3 )δ(ppm): 1.42(-C(CH 3 ) 2 ), 3.43-3.83 (-CH 2 CH 2 O-, glycidol, -OCHCH 2 O...
preparation example 2
[0041] Preparation Example 2: Preparation of Compound 2
[0042] Add about 18.8g (Mn=6,000g / mol, 3.13mmol) of compound 1 (polyethylene glycol with end group protection) into a clean 250mL round bottom flask, and add 150mL THF to dissolve it, then add 13mL concentrated hydrochloric acid (Concentration is 37wt%), after stirring at room temperature for 6 hours, slowly add potassium hydroxide to adjust the pH value to 7.0, filter and separate the salt generated, and precipitate the solvent evaporated in the filtrate twice in ether, and the precipitate is at 45 After vacuum drying at °C for 12 hours, polyethylene glycol (compound 2) with two "active" hydroxyl groups at the end was obtained. Compound 2 1 H NMR is: 1 H NMR (CDCl 3 )δ(ppm): 1.42(-C(CH 3 ) 2 ), 3.43-3.84 (-CH 2 CH 2 O-, glycidol, -OCHCH 2 O-), 5.01 (-OCH 2 Ph), 7.16-7.23 (-C 6 h 5 ). The number average molecular weight was 6000. The molecular weight distribution was 1.01.
preparation example 3
[0043] Preparation Example 3: Preparation of Compound 3
[0044] Under a nitrogen atmosphere, potassium hydride (201 mg, 5.3 mmol), tetrahydrofuran solution (125 mL), and compound 2 (15 g, 2.5 mmol) were successively added to a 250 mL dry and clean double-neck round bottom flask, and stirred at room temperature for four hours. Subsequently, ethylene oxide (30 g, 682 mmol) was added through a constant pressure dropping funnel in an anhydrous and oxygen-free system and reacted at room temperature for 36 hours. Add excessive methyl iodide then and active oxygen anion is capped, continue to react at 50 ℃ for 24 hours. The generated salt was separated by centrifugation, and then the solvent of the supernatant was evaporated by rotary evaporation, and precipitated in diethyl ether, and the precipitate was dried in a vacuum oven at 45° C. for 12 hours to obtain compound 3. Compound 3 1 H NMR is: 1 H NMR (CDCl 3 )δ(ppm): 1.43(-C(CH 3 ) 2 ), 3.43-3.86 (-CH 2 CH 2 O-, glycidol, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com