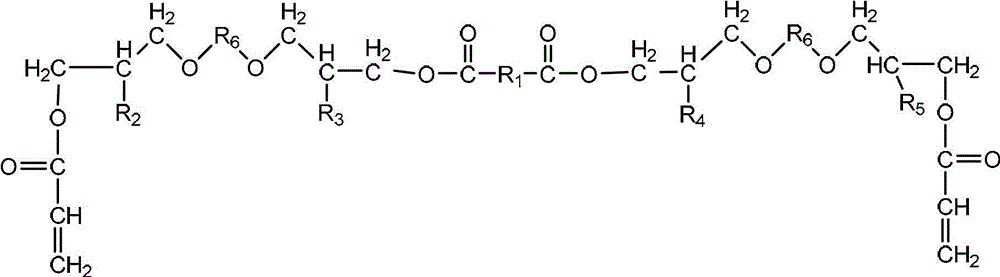
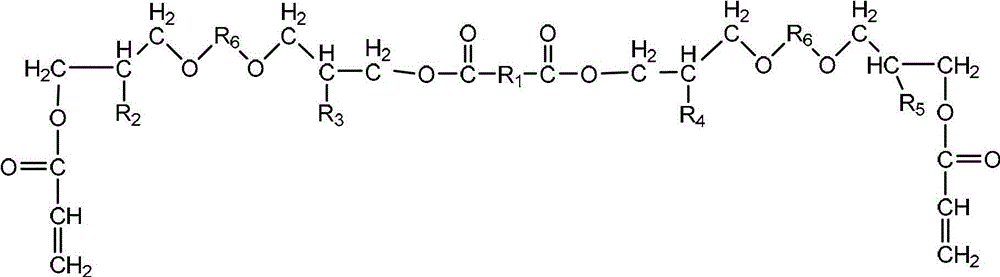
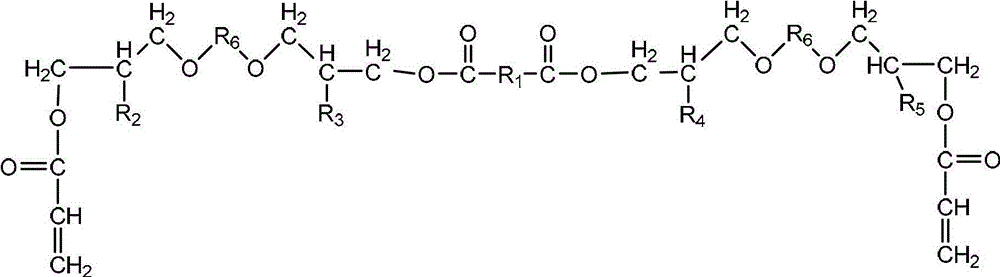
Multifunctional urethane acrylate oligomer as well as synthesis method and application thereof
A technology of polyurethane acrylate and synthesis method, applied in the direction of polyurea/polyurethane coatings, coatings, etc., can solve the problems of increasing the brittleness of the coating film and decreasing the adhesion, and achieve the effects of improving the brittleness, less side reactions, and simple synthesis route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Synthesis of modified glycidyl ether
[0035] Add 1,4-cyclohexanedimethanol glycidyl ether, adipic acid and catalyst triphenylphosphine into the reactor and raise the temperature to form a homogeneous phase. Control the temperature at 120°C for 2.5 hours until the acid value drops to the initial acidity of the system. When the acid value is 3% of the initial acid value, the reaction is stopped; the temperature is lowered to 90 ° C, and then the acrylic acid solution dissolved with the polymerization inhibitor hydroquinone is added dropwise, and the reaction is carried out for 3.0 hours. When the acid value is reduced to 3% of the initial acid value, the reaction is stopped, and the reaction obtains Modified glycidyl ether (A) containing 4 hydroxyl groups and two double bonds in the molecular structure; the molar ratio of 1,4-cyclohexanedimethanol glycidyl ether to adipic acid is 2:1, acrylic acid to 1, The molar ratio of 4-cyclohexanedimethanol glycidyl ether is 1.0...
Embodiment 2
[0050] (1) Synthesis of modified glycidyl ether
[0051] Add ethylene glycol diglycidyl ether, sebacic acid and catalyst triphenylphosphine into the reactor to raise the temperature to form a homogeneous phase, control the temperature at 140°C for 3.0 hours, until the acid value drops below 2% of the initial acid value of the system stop the reaction; lower the temperature to 100°C, then dropwise add the acrylic acid solution dissolved in the polymerization inhibitor p-hydroxyanisole, react for 2.5h, stop the reaction when the acid value drops below 1% of the initial acid value, and obtain the molecular structure Modified glycidyl ether (A) containing 4 hydroxyl groups and two double bonds; the molar ratio of polyethylene glycol diglycidyl ether to sebacic acid is 2.05:1, acrylic acid and polyethylene glycol diglycidyl ether The molar ratio is 1.05: 1, the consumption of catalyst triphenylphosphine is 0.6% of the total mass of polyethylene glycol diglycidyl ether and sebacic a...
Embodiment 3
[0060] (1) Synthesis of modified glycidyl ether
[0061] Add propylene glycol diglycidyl ether, succinic acid and catalyst triphenylphosphine into the reactor to raise the temperature to form a homogeneous phase, control the temperature at 145°C for 2.5 hours, and stop when the acid value drops below 1% of the initial acid value of the system Reaction; lower the temperature to 105°C, then dropwise add an acrylic acid solution in which the polymerization inhibitor hydroquinone is dissolved, react for 3.0 hours, and stop the reaction when the acid value is reduced to less than 3% of the initial acid value, and the reaction obtains a molecular structure containing 4 A modified glycidyl ether (A) with two hydroxyl groups and two double bonds; the molar ratio of polypropylene glycol diglycidyl ether to succinic acid is 2.05:1, and the molar ratio of acrylic acid to polypropylene glycol diglycidyl ether is 1.1:1 , the consumption of catalyst triphenylphosphine is 0.8% of the total m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com