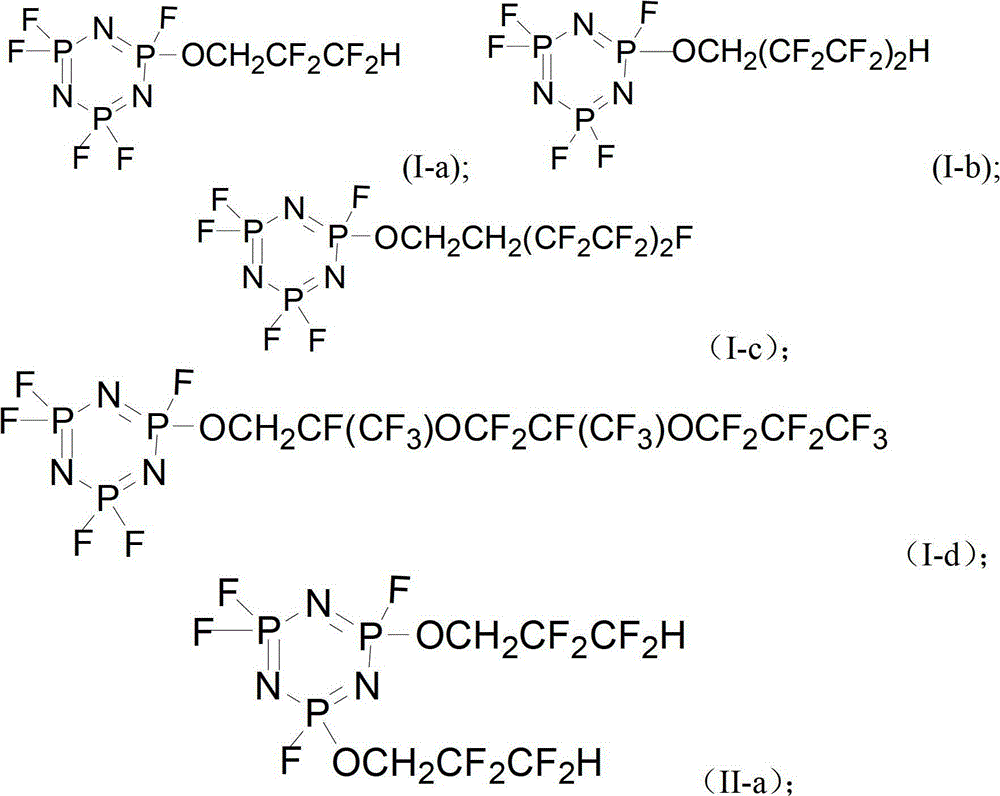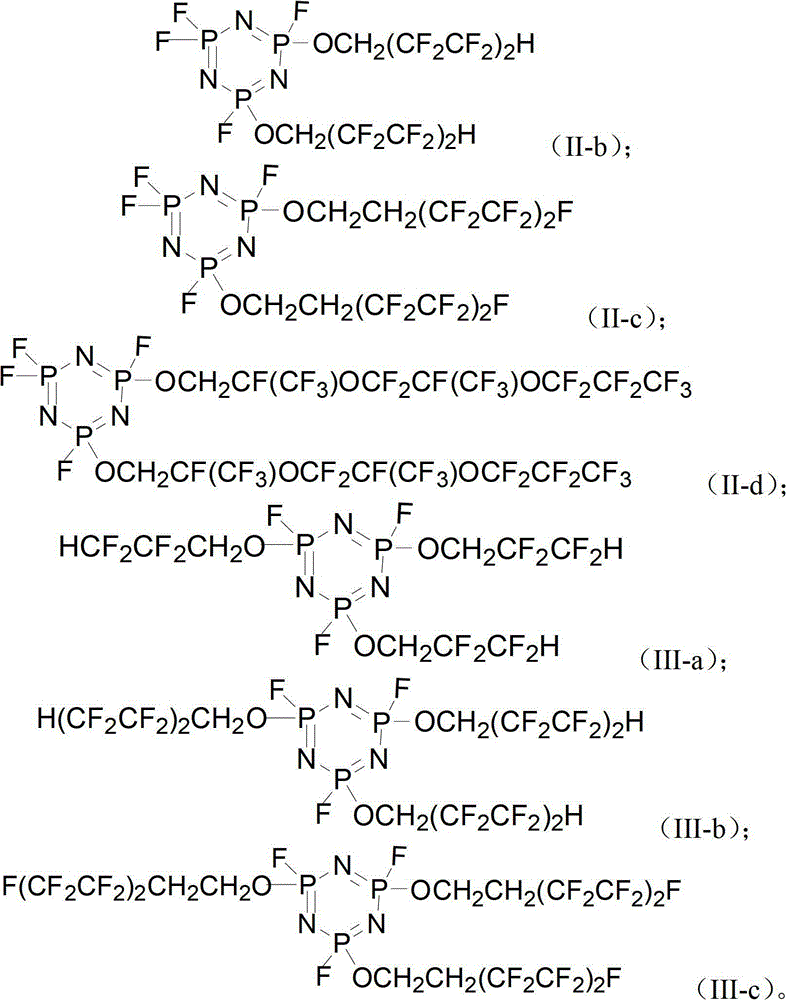Phosphazene flame retardant, preparation method thereof and lithium-ion battery electrolyte
A phosphazene flame retardant, lithium ion battery technology, applied in secondary batteries, chemical instruments and methods, secondary battery repair/maintenance, etc., can solve battery capacity loss, flame retardant viscosity, system compatibility problems such as poor performance, to achieve the effect of enhancing stability, improving flame retardant performance, and improving electrical conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] Correspondingly, the present invention also provides a preparation method of a phosphazene flame retardant, comprising the following steps:
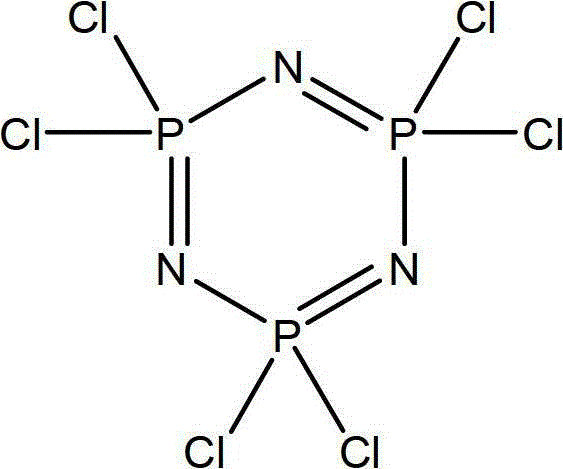
[0043] Reaction of fluorocarbon alcohol having the structure of formula (IV) with hexachlorocyclotriphosphazene to obtain an intermediate product;
[0044] Rf-OH (IV);
[0045] Wherein, Rf is a fluorine-substituted alkyl group or a fluorine-substituted polyether;
[0046] The intermediate product and HF are fluorinated to obtain a phosphazene flame retardant, and the phosphazene flame retardant has a structure of (I), formula (II) or formula (III):
[0047] Formula (I); Formula (II);
[0048] Formula (III);
[0049] Wherein, Rf is a fluorine-substituted alkyl group or a fluorine-substituted polyether.
[0050] The fluorocarbon alcohol has the structure of formula (IV), wherein Rf is a fluorine-substituted alkyl or a fluorine-substituted polyether, preferably formula (IV-1), formula (IV-2) or formula (IV-3) Compounds sho...
Embodiment 1
[0089] Dissolve 26.4g (0.2mol) of tetrafluoropropanol in 52.8mL of tetrahydrofuran, add 5.04g (0.21mol) of NaH and react under reflux for 5h to obtain sodium tetrafluoropropoxide solution. in N 2 Slowly drop the above sodium tetrafluoropropoxide solution into 69.6g (0.2mol) of hexachlorocyclotriphosphazene in 69.6mL of tetrahydrofuran solution under the protection of the protection of 12h, reflux for 12h, filter, wash with tetrahydrofuran, and the filtrate is washed with anhydrous magnesium sulfate After drying, tetrahydrofuran was removed by atmospheric distillation, and finally 78.2 g of products containing tetrafluoropropyl ether-pentachlorocyclotriphosphazene were obtained by distillation under reduced pressure, with a yield of 88.3%. Among them, tetrafluoropropyl ether-pentachlorocyclotriphosphazene The purity is 95%.
[0090] Add 44.3g (0.1mol) of tetrafluoropropyl ether-pentachlorocyclotriphosphazene and 300g (1.5mol) of anhydrous HF prepared above into a 500mL Monel s...
Embodiment 2
[0095] Get octafluoropentanol 6.4g (0.2mol) and dissolve in 92.8mL tetrahydrofuran, add 5.04g (0.21mol) NaH, the reaction process is the same as in Example 1, obtain the octafluoropentyl-pentachlorocyclotriphosphazene 96.2 g of the product, the yield is 88.6%, and the purity of octafluoropentyl-pentachlorocyclotriphosphazene is 95%.
[0096] 54.3g (0.1mol) of the above-prepared octafluoropentyl-pentachlorocyclotriphosphazene and 300g (1.5mol) of anhydrous HF, the reaction process was the same as in Example 1, and 42.0g of the product was obtained, with a yield of 91.2%. .
[0097] Use NMR to analyze and identify the product, the results are as follows: 1HNMR (400MHz, CDCl3), δ: 3.91(m, 2H, -CH 2 -);δ:5.70(m,1H,-CF 2 H-). 19F NMR (400MHz, CDCl3), δ: -71 (4F); δ: -60 (1F); δ: -138 (2F); δ: -126 (4F); δ: -123 (2F).
[0098] The results showed that the obtained product was octafluoropentyl-pentafluorocyclotriphosphazene with the structure (I-b), and its purity was 99%.
[009...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com