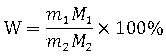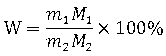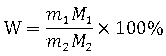Method for analyzing lithium ion battery electrolyte salt LiBF4
A lithium-ion battery and electrolyte salt technology, applied in the field of analytical chemistry, can solve problems such as unpublished industry measurement methods by enterprises, and achieve the effect of overcoming error increase and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] A kind of lithium ion battery electrolyte salt LiBF 4 Analytical method, including the following steps:
[0022] ⑴ Prepare and calibrate Na 2 S 2 o 3 .5H 2 O solution: 10g Na 2 S 2 o 3 ·5H 2 O was dissolved in 800mL water, boiled on an electric stove for 10min, and left at room temperature to obtain a concentration of 5×10 -2 ~6×10 -2 mol / L Na 2 S 2 o3 .5H 2 O; using benchmark reagent K 2 Cr 2 o 7 The solution was prepared according to the conventional method for Na 2 S 2 o 3 .5H 2 O for calibration, that is, 0.056mol / L Na 2 S 2 o 3 ·5H 2 O solution standard solution.
[0023] ⑵ Prepare and calibrate KI 3 Solution: Dissolve 15.0 g of KI in water, then add 6.4 g of I 2 After it is completely dissolved, transfer it into a 500mL brown bottle, add water to dilute to the mark, and leave it for 24~48h to get KI 3 solution; pipette 10.00mL of the Na obtained in step ⑴ 2 S 2 o 3 ·5H 2 O solution standard solution in the iodine bottle, diluted with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com