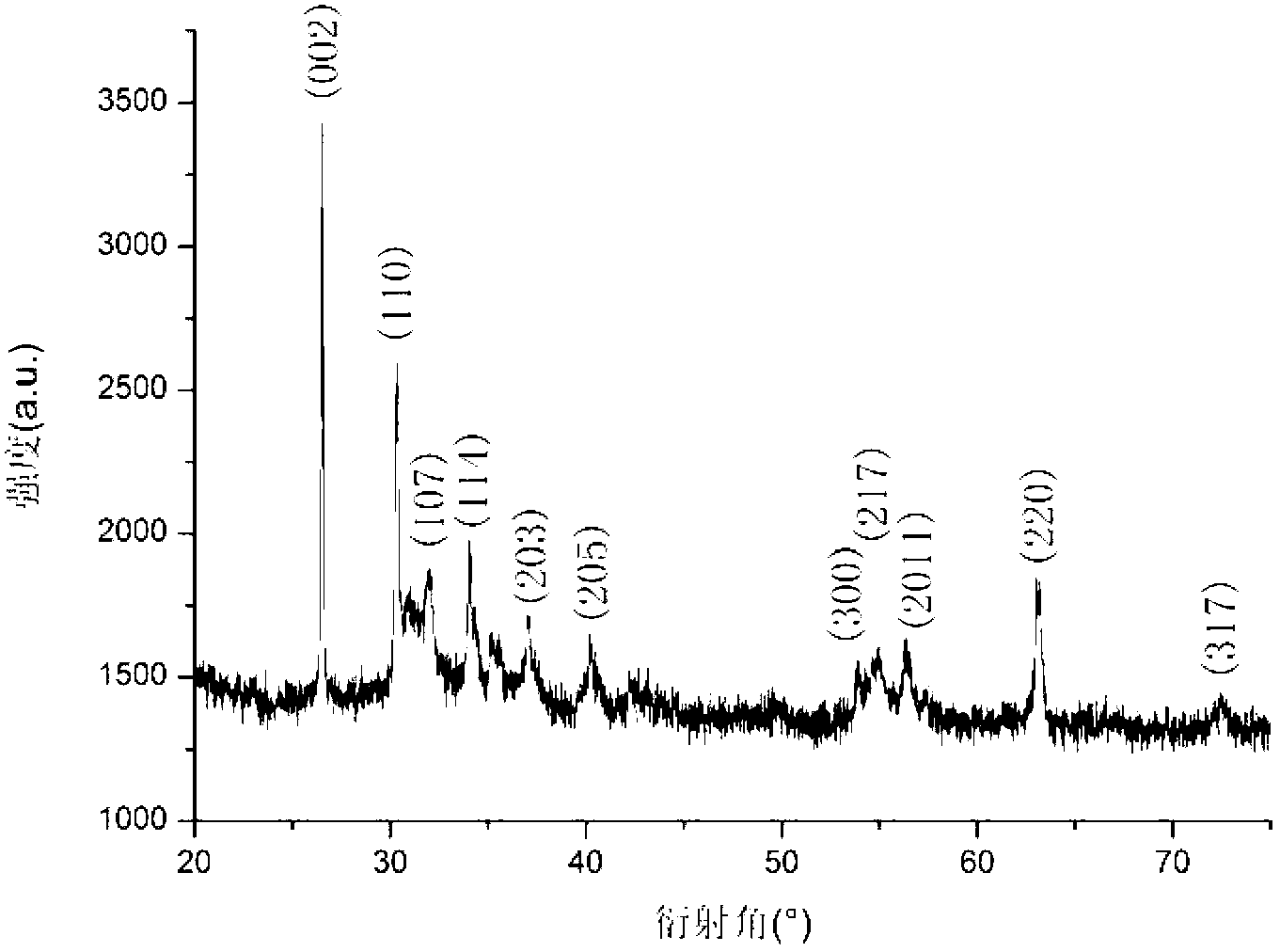
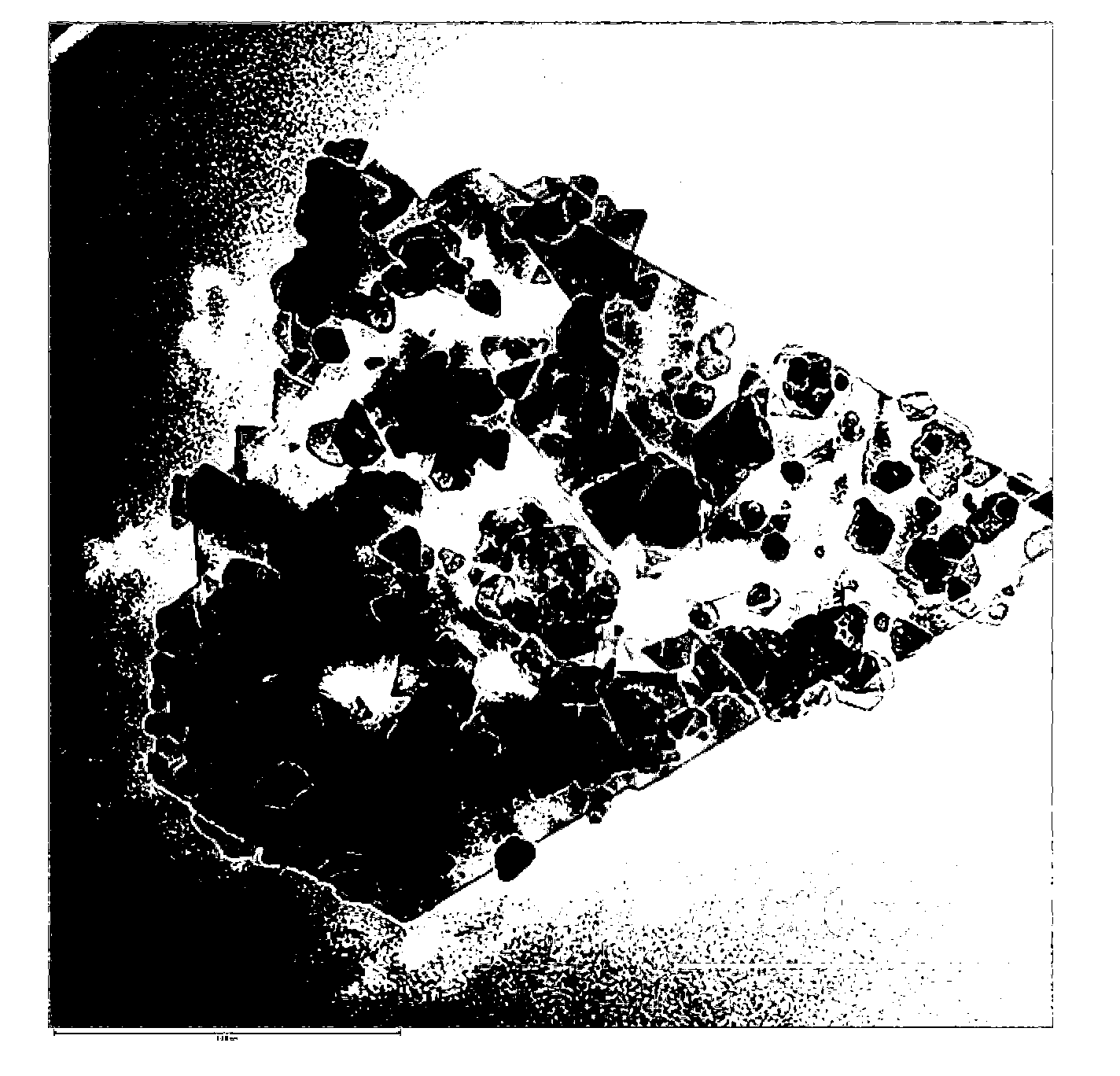
Preparation method of composite material of graphite flake-load barium ferrite nanoparticles
A nanoparticle and composite material technology, applied in the field of composite magnetic material preparation, can solve the problems of expensive raw carbon nanotubes, easy to be oxidized, poor chemical stability, etc., and achieve the effects of reducing chemical reaction energy, reducing synthesis temperature, and uniform distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (A). 5.94g of FeCl 3 ·6H 2 O (analytical pure) was placed in a beaker, and 50mL of deionized water was added to dissolve it completely, and then 5.5mL of concentrated ammonia water (analytical pure) solution was slowly added dropwise under strong stirring, and after standing for aging, the upper layer After absorbing the suspended impurities, add 0.006mol of barium acetate (analytical pure) solution and stir evenly;
[0023] (B). Add 44g of sodium hydroxide (analytically pure) to the mixed solution prepared in step (A), and add a certain amount of absolute ethanol solution, then stir to make it completely dissolve, and the added absolute ethanol The volume is 20% of the total volume of the mixed solution, and the total volume of the mixed solution must not exceed 150mL;
[0024] (C). Add 10g of natural graphite into the mixed solution of concentrated nitric acid and concentrated hydrochloric acid, wherein the concentrated nitric acid is 150mL, and the concentrated hyd...
Embodiment 2
[0031] (A). Add 6.5g of FeCl 3 ·6H 2 O (analytical pure) was placed in a beaker, and 50mL of deionized water was added to dissolve it completely. Then, under strong stirring, 6mL of concentrated ammonia water (analytical pure) solution was slowly dropped into it. After standing for aging, the upper layer was suspended. After absorbing the impurities, add 0.006mol of barium acetate (analytical grade) solution and stir evenly;
[0032] (B). Add 46g of sodium hydroxide (analytically pure) to the mixed solution prepared in step (A), and add a certain amount of absolute ethanol solution, then stir to make it completely dissolve, and the added absolute ethanol The volume is 20% of the total volume of the mixed solution, and the total volume of the mixed solution must not exceed 160mL;
[0033](C). Add 10g of natural graphite into the mixed solution of concentrated nitric acid and concentrated hydrochloric acid, wherein the concentrated nitric acid is 150mL, and the concentrated hy...
Embodiment 3
[0038] (A). 5.6g of FeCl 3 ·6H 2 O (analytical pure) was placed in a beaker, and 50mL of deionized water was added to dissolve it completely. Then, under strong stirring, 5mL of concentrated ammonia water (analytical pure) solution was slowly dropped into it. After standing for aging, the upper layer was suspended. After absorbing the impurities, add 0.006mol of barium acetate (analytical grade) solution and stir evenly;
[0039] (B). Add 42g of sodium hydroxide (analytical pure) to the mixed solution prepared in step (A), and add a certain amount of absolute ethanol solution, then stir to make it completely dissolve, and the added absolute ethanol The volume is 20% of the total volume of the mixed solution, and the total volume of the mixed solution must not exceed 150mL;
[0040] (C). Add 10g of natural graphite into the mixed solution of concentrated nitric acid and concentrated hydrochloric acid, wherein the concentrated nitric acid is 150mL, and the concentrated hydroch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com