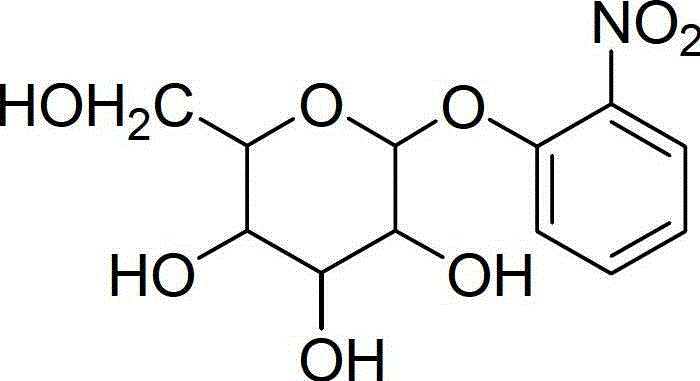
Preparation method of o-nitrobenzene galactoside
A technology of o-nitrophenyl galactoside and nitrobenzene half, which is applied in the field of sugar engineering, can solve the problems of low yield and incomplete reaction of glycoside products, and achieve the effect of increasing reaction yield and shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The invention provides a preparation method of o-nitrophenylgalactoside, comprising:
[0022] A) Tetraacetylbromogalactose, o-nitrophenol, sodium hydroxide and tetrabutylammonium bromide are heated and reacted in water and dichloromethane to obtain tetraacetyl o-nitrophenylgalactoside;
[0023] B) Deacetylation of tetraacetyl-o-nitrophenylgalactoside to obtain o-nitrophenylgalactoside.
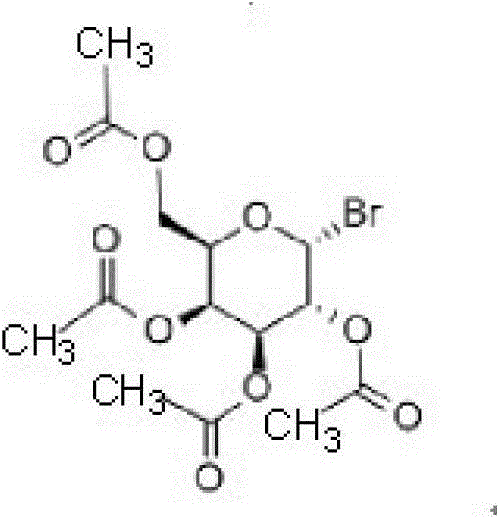
[0024] The present invention uses tetraacetylbromogalactose as the raw material, which has the structure of formula (II), and the present invention has no special limitation on its source, preferably commercially available;
[0025]
[0026] Formula (II).
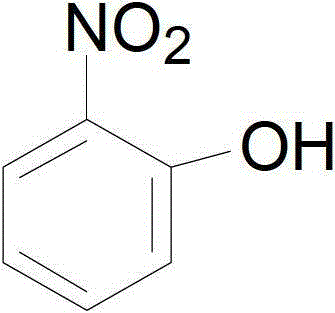
[0027] The present invention uses o-nitrophenol as a raw material, which has a structure of formula (III), and the present invention has no special limitation on its source, preferably commercially available;
[0028]
[0029] Formula (III).
[0030] The present invention has no special limitation on the source of sodium hyd...
Embodiment 1
[0042] Stir and dissolve 1 mol of tetraacetylbromogalactose and 20 mol of dichloromethane, add 1 mol of tetrabutylammonium bromide, 1 mol of sodium hydroxide, 20 mol of water, and 1 mol of o-nitrophenol into the reaction kettle, reflux at 40.2°C After reacting for 2 hours, a reaction solution was obtained, and thin layer chromatography (TLC) showed that the reaction was complete. The organic phase was separated from the reaction solution, evaporated to dryness, recrystallized by adding 1.5 mol of ethanol, and dried to obtain 0.876 mol of tetraacetyl-o-nitrophenylgalactoside.
[0043] Add 0.876 mol of tetraacetyl-o-nitrophenylgalactoside prepared above, 1.5 mol of methanol, and 0.01 mol of sodium methoxide into the reaction kettle, stir and react at room temperature for 2 hours to obtain a reaction liquid, which is detected by TLC and the reaction is complete. Add 0.01 mol of acetic acid to neutralize the reaction solution, evaporate to dryness, filter and dry to obtain 0.781 m...
Embodiment 2
[0046] Stir and dissolve 1 mol of tetraacetylbromogalactose and 20 mol of dichloromethane, add 1 mol of tetrabutylammonium bromide, 1.1 mol of sodium hydroxide, 20 mol of water, and 1 mol of o-nitrophenol into the reaction kettle, and condense at 40.2°C Reflux reaction for 2 hours to obtain a reaction solution, TLC showed that the reaction was complete. Separate the organic phase from the reaction solution, evaporate the organic phase to dryness, add 1.5 mol of ethanol, recrystallize and dry to obtain 0.882 mol of tetraacetyl-o-nitrophenylgalactoside.
[0047] Add 0.882 mol of tetraacetyl-o-nitrophenylgalactoside prepared above, 1.5 mol of methanol, and 0.01 mol of sodium methoxide into the reaction kettle, stir and react at room temperature for 2 hours, and obtain the reaction solution. After TLC detection, the reaction is complete. Add 0.01 mol of acetic acid neutralized the reaction liquid, evaporated to dryness, filtered, and dried to obtain 0.783 mol of the reaction produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com