Acetazolamide sustained-release capsule and preparation method thereof
A technology of acetazolamide and sustained-release capsules, which is used in pharmaceutical formulations, medical preparations containing active ingredients, and drug delivery, etc., can solve the problems of high peak blood drug concentration, too fast release rate of common dosage forms, and inability to be replaced. , to achieve the effect of less toxic and side effects, reducing toxic and side effects, and facilitating long-term treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
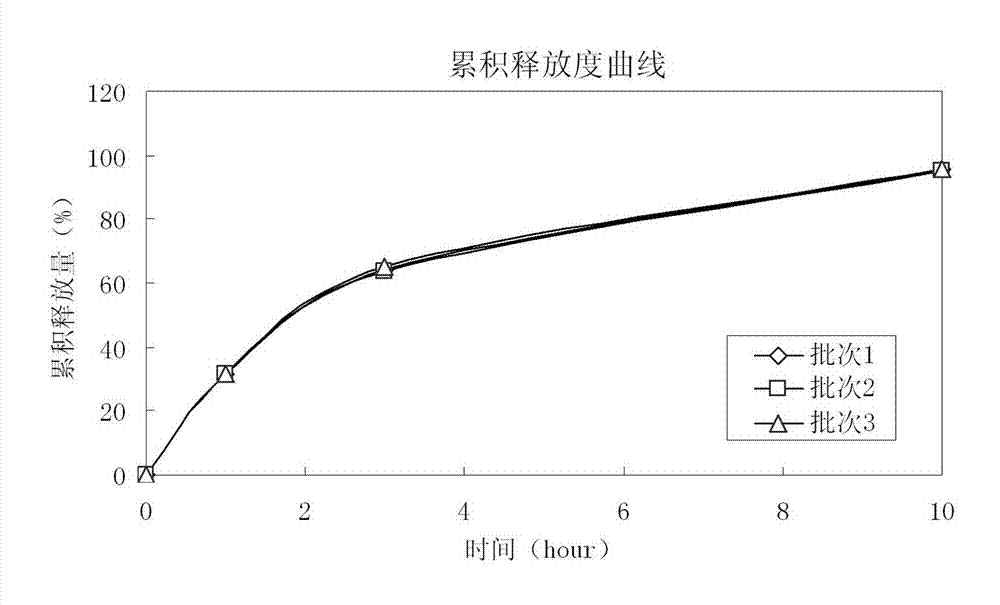
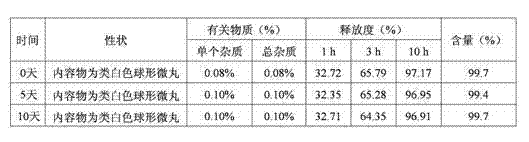
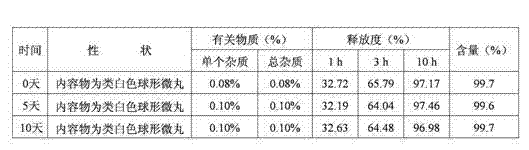
[0047] The raw and auxiliary materials of the acetazolamide sustained-release capsule of the present invention are composed of: according to the composition formula of the raw and auxiliary materials, the expansion is 6.3 times, expressed in parts by weight g, consisting of 500 g of acetazolamide, 130 g of microcrystalline cellulose, and 2.0 g of sodium lauryl sulfate And purified water 230g composition.
[0048] Explanation: The water in the adhesive added in this example goes through the preparation method and is finally dried to obtain the product. The added water is all evaporated, so the proportion of the above raw and auxiliary materials is calculated as solid matter.
[0049] The preparation method of acetazolamide sustained-release capsules of the present invention:
[0050] a. Prepare various raw and auxiliary materials according to the composition of the raw and auxiliary materials of the above-mentioned acetazolamide sustained-release capsules, dissolve the auxiliar...
Embodiment 2
[0052] Embodiment 2: basically the same as Embodiment 1, the difference is:
[0053] The raw and auxiliary materials of the acetazolamide sustained-release capsule of the present invention are composed of: according to the composition formula of the raw and auxiliary materials, the expansion is 6.4 times, expressed in parts by weight g, consisting of 500 g of acetazolamide, 125 g of microcrystalline cellulose, and 1.5 g of sodium lauryl sulfate , 6.3g of talcum powder and 225g of 2% hypromellose aqueous solution in mass percent concentration.
[0054] Explanation: The water in the adhesive added in this example goes through the preparation method and is finally dried to obtain the product. The added water is all evaporated, so the proportion of the above raw and auxiliary materials is calculated as solid matter.
[0055] The preparation method of the acetazolamide sustained-release capsule of the present invention is the same as in Example 1.
Embodiment 3
[0056] Embodiment 3: basically the same as Embodiment 1, the difference is:
[0057] The raw and auxiliary materials of the acetazolamide sustained-release capsule of the present invention are composed of: according to the composition formula of the raw and auxiliary materials, the expansion is 6.9 times, expressed in parts by weight g, consisting of 525g of acetazolamide, 110g of microcrystalline cellulose, and 1.0g of sodium lauryl sulfate , 50g of sucrose, 5g of talcum powder and 200g of purified water.
[0058] Explanation: The water in the adhesive added in this example is prepared through the preparation method and finally dried to obtain the product. The added water is all evaporated, so the proportion of the above raw and auxiliary materials is calculated as solid matter;
[0059] The preparation method of the acetazolamide sustained-release capsule of the present invention is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com