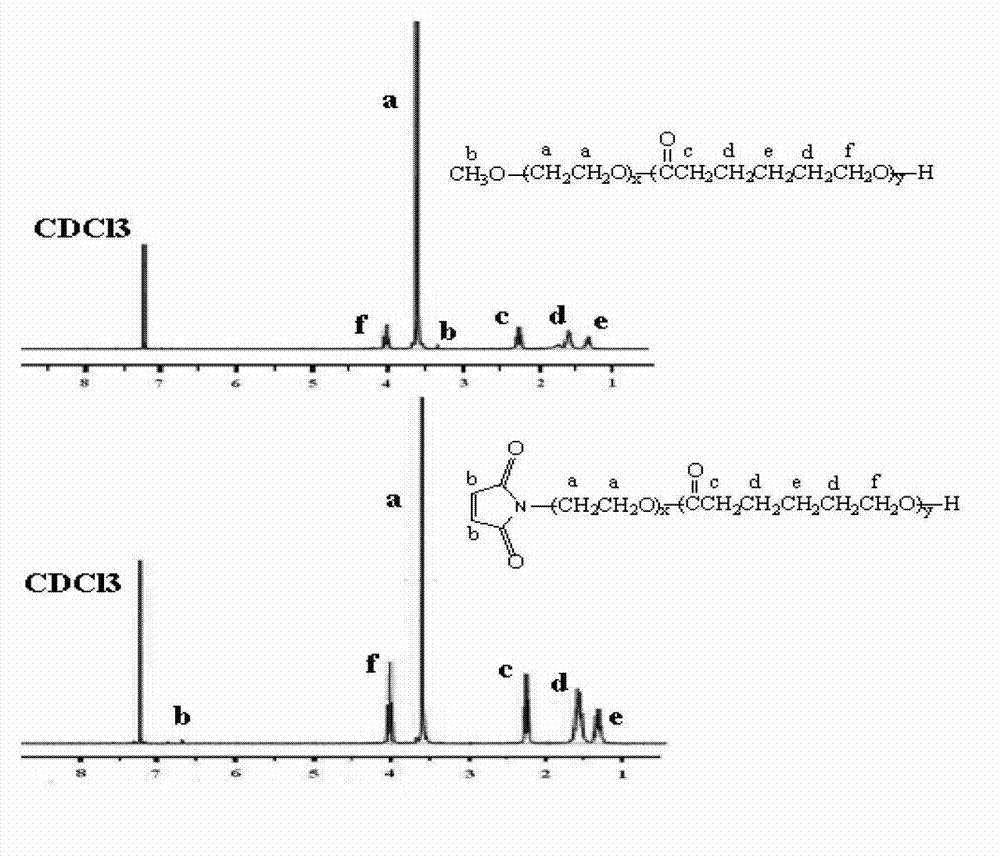
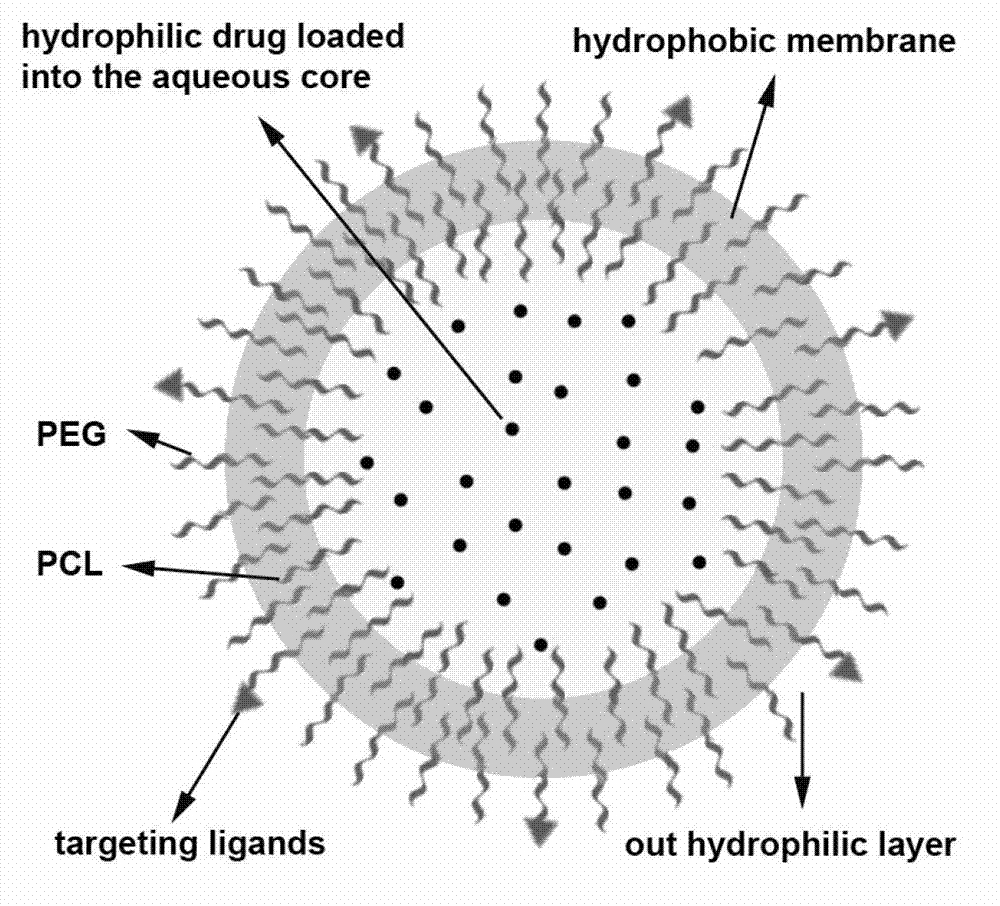
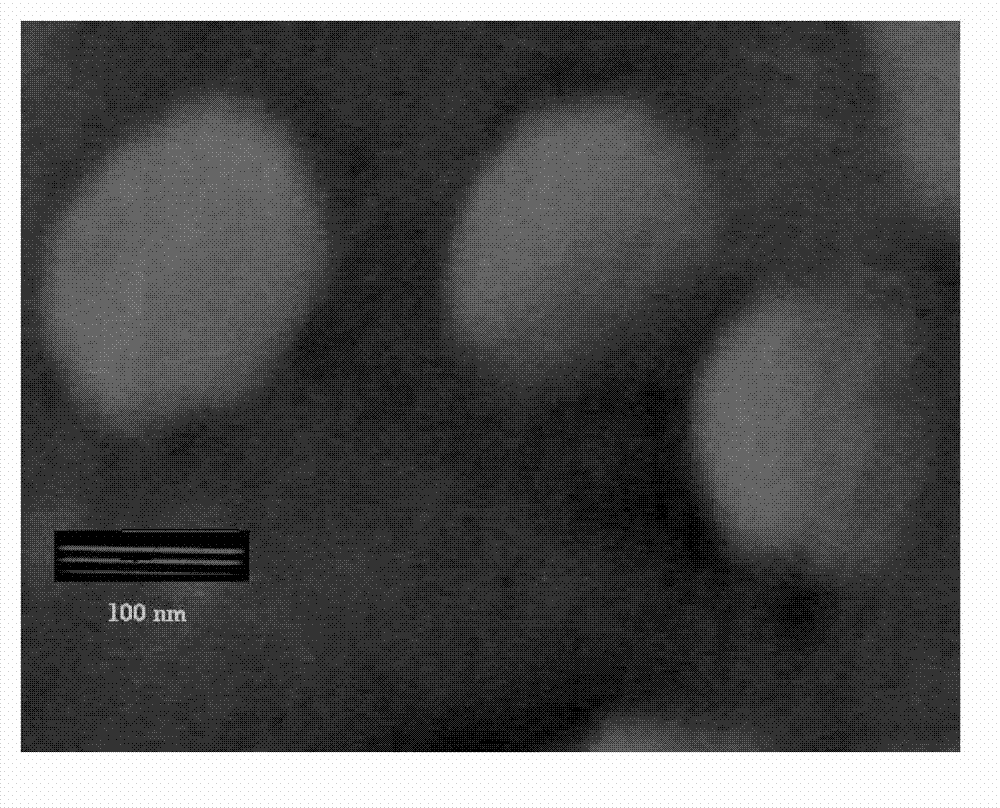
Oxymatrine hepatic targeting nano drug delivery system and preparation method thereof
A technology of oxymatrine and drug delivery system, which is applied in the field of nano-targeted drug delivery system and preparation containing oxymatrine, can solve the problems of increased dose, less liver distribution, short half-life of oxymatrine, and the like, Achieving the effect of delaying clearance, improving efficacy, and increasing access to the liver
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 film dispersion method
[0034]Put MALPEG3500-PCL9000 and MPEG3000-PCL4000 in a molar ratio of 1:10~15 into a 50ml round bottom flask, add 1~3ml of chloroform to dissolve, heat in a water bath at 32~38°C to remove the organic solvent, and then pass it into Nitrogen for 2 to 5 minutes to remove residual solvent to form a dry polymer film; add 2ml of citric acid buffer (pH: 3.5 to 4.5) with a concentration of 150mM / L, transfer the suspension to a 5ml beaker, seal it, and stir at 45 to 70°C Hydrate for 4-8 hours, then ultrasonically disperse for 5-15 minutes with a power of 70-150w, and obtain blank polymer nanoparticles with a particle size of about 80-120nm; after adjusting the pH to 3.5-5 with 1M / L sodium hydroxide, press oxidation The mass ratio of matrine: polyethylene glycol-polycaprolactone is 1:3~8. Add the stock solution of oxymatrine to the system obtained in the step, stir and incubate in a water bath at 25~65°C for 1~3h, and then use Sodium hydrox...
Embodiment 2
[0035] Embodiment 2 film dispersion method
[0036] Put MALPEG4500-PCL1000 and MPEG5000-PCL9000 in a molar ratio of 1:10~15 into a 50ml round bottom flask, add 1~3ml of chloroform to dissolve, heat in a water bath at 32~38°C to remove the organic solvent, and then pass it into Nitrogen for 2 to 5 minutes to remove residual solvent to form a dry polymer film; add 2ml of citric acid buffer (pH: 3.5 to 4.5) with a concentration of 150mM / L, transfer the suspension to a 5ml beaker, seal it, and stir at 55 to 75°C Hydrate for 6-12 hours, and then ultrasonically disperse with 200-400w power for 8-20 minutes to obtain blank polymer nanoparticles with a particle size of about 100-160nm; after adjusting the pH to 3.5-5 with 1M / L sodium hydroxide, press oxidation The mass ratio of matrine: polyethylene glycol-polycaprolactone is 1:3~8. Add the oxymatrine stock solution to the system obtained in the step, stir and incubate in a water bath at 25~65°C for 2~5h, and then use Sodium hydroxid...
Embodiment 3
[0037] Embodiment 3 reverse evaporation method
[0038] Dissolve MALPEG3.5K-PCL7K and MPEG3K-PCL4K in a molar ratio of 1:10-15 in 2-3 mL of chloroform to form an organic phase; Dilute the oxymatrine stock solution into 3-5 mL of 0.05 M / L phosphate buffer solution to form an aqueous phase. Ultrasonic the organic phase at a power of 100-150W, add the water phase to the organic phase, ultrasonically form a uniform dispersion system of water and oil, and remove the organic solvent by rotary evaporation under reduced pressure in a water bath at 40-60°C to obtain Shake the colloidal solution and continue to evaporate under reduced pressure to obtain drug-loaded polymer nanoparticles with a particle size of about 100-180°C and uniform size distribution. Use 1M / L sodium hydroxide to adjust the pH to 7.35-7.45; Peptide RGD was added to the drug-loaded nanoparticles according to the molar ratio of RGD:MALPEG-PCL 1:5~15, and after 10~12 hours of magnetic stirring reaction at 25~37°C, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com