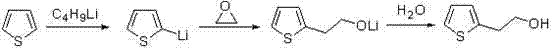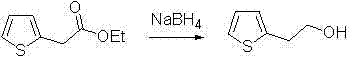Process for synthesizing 2-thiopheneethanol and derivatives thereof
A synthesis method, the technology of thiophene ethanol, which is applied in the field of medicine and chemical industry, can solve the problems of easy volatilization of bromine, many steps, cumbersome reaction, etc., and achieve the effect of reducing synthesis steps, mild reaction conditions, and avoiding formative reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1) Preparation of the compound of formula 1: Under the protection of nitrogen, dissolve 33g of 2-bromothiophene, 48g of the compound of formula 4, 18g of sodium acetate, and 2.3g of palladium acetate in 200ml of N-methylpyrrolidone, stir and heat up to 135°C , kept warm for 9 hours, cooled to room temperature after the reaction, quenched by adding 200ml ice water, extracted 200mlⅹ2 with toluene, washed the organic phase with water 200mlⅹ2, then dried overnight with anhydrous sodium sulfate, filtered, and distilled under reduced pressure to obtain a viscous solid , adding 100ml of anhydrous methanol for recrystallization to obtain 48.7g of the compound of formula 1 as a white solid, with a yield of about 86%.
[0046] (2) Preparation of the compound of formula 2: In an organic solvent, 2.4 g of Pd / C (Pd mass fraction 10%) was added to 48.7 g of the compound of formula 1, and the 2 After replacing the air, the reaction pressure is 1-1.2MPa, and after stirring at 45-50°C ...
Embodiment 2
[0050] (1) Preparation of the compound of formula 1: Under the protection of nitrogen, dissolve 33g of 2-bromothiophene, 48g of the compound of formula 4, 18g of sodium acetate, and 2.3g of palladium acetate in 200ml of N-methylpyrrolidone, stir and heat up to 135°C , kept warm for 9 hours, cooled to room temperature after the reaction, quenched by adding 200ml ice water, extracted 200mlⅹ2 with toluene, washed the organic phase with water 200mlⅹ2, then dried overnight with anhydrous sodium sulfate, filtered, and distilled under reduced pressure to obtain a viscous solid , adding 100ml of anhydrous methanol for recrystallization to obtain 48.7g of the compound of formula 1 as a white solid, with a yield of about 86%.
[0051] (2) Preparation of the compound of formula 2: in an organic solvent, add 4.8 g of Pd / C (Pd mass fraction 10%) to 48.7 g of the compound of formula 1, and use H 2 After replacing the air, the reaction pressure was 1.0-1.2MPa, and after stirring at 45-50°C f...
Embodiment 3
[0055] (1) Preparation of compound of formula 1: Under nitrogen protection, dissolve 33g of 2-bromothiophene, 48g of compound of formula 4, 18g of sodium acetate, 4.6g of palladium acetate in 200ml of N-methylpyrrolidone, stir and heat up to 135°C , kept warm for 9 hours, cooled to room temperature after the reaction, quenched by adding 200ml ice water, extracted 200mlⅹ2 with toluene, washed the organic phase with water 200mlⅹ2, then dried overnight with anhydrous sodium sulfate, filtered, and distilled under reduced pressure to obtain a viscous solid , adding 100ml of anhydrous methanol for recrystallization to obtain 50.5g of the compound of formula 1 as a white solid, with a yield of about 89%.
[0056] (2) Preparation of the compound of formula 2: in an organic solvent, add 2.5 g of Pd / C (Pd mass fraction 10%) to 50.5 g of the compound of formula 1, and use H 2 After replacing the air, the reaction pressure was 1.0-1.2MPa, and after stirring at 45-50°C for 5 hours, the Pd / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com