Method for preparing high-purity cefdinir antibiotic 7-side chain synthesis critical material
A technology of cefdinir and antibiotics, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of affecting the yield, easy leakage during use, cumbersome handling, etc., and achieves the effects of convenient and safe transportation, easy recycling and good reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
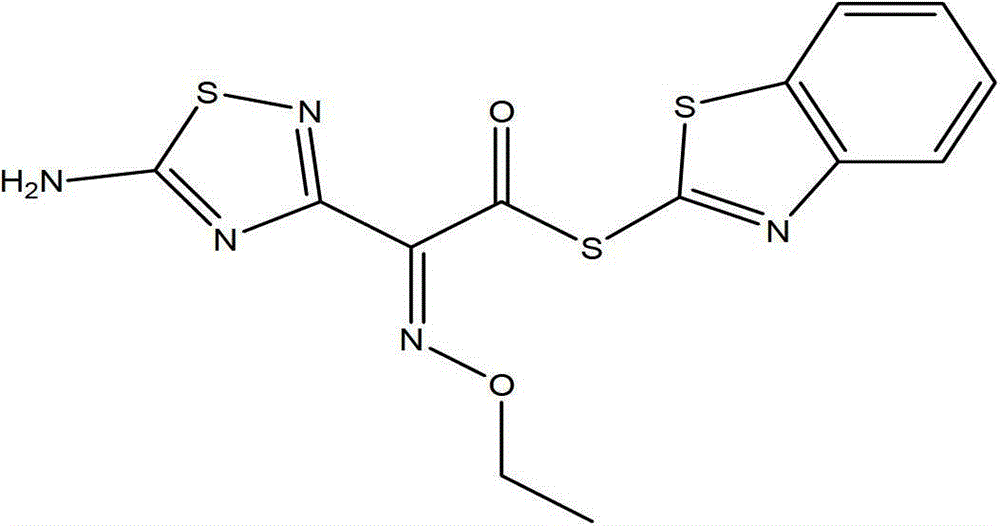
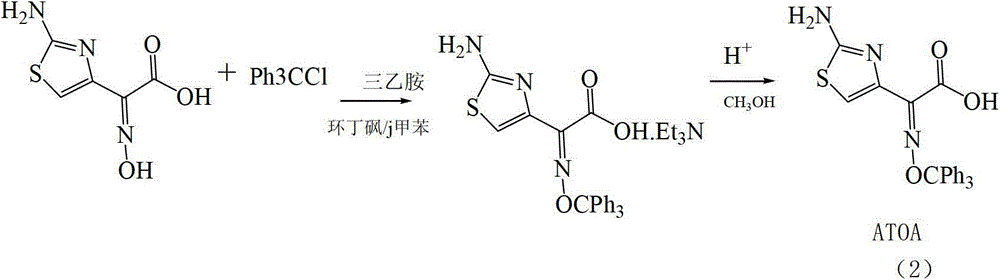
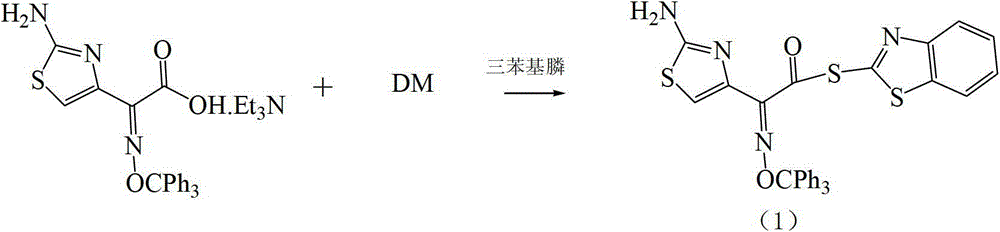
[0058] A method for preparing key raw materials for the synthesis of high-purity cefdinir antibiotic 7-side chain, including three reaction steps of alkylation, hydrolysis and esterification.
[0059] (A) Alkylation reaction is carried out at 20-40°C, using desmethylaminothioxamic acid ethyl ester and triphenylmethanol as raw materials, carbonate solvent as solvent, and dimethyl carbonate boron trifluoride solid complex (Z)-2-(2-amino-4-thiazolyl)-2-trityloxyimine ethyl acetate (compound 3). Wherein, carbonate solvent comprises a kind of in dimethyl carbonate, diethyl carbonate, preferably selects dimethyl carbonate, and the consumption of carbonate solvent is 3~20 times of desmethylaminothiaxamic acid ethyl ester by weight, Preferably 5 times.
[0060] The reaction formula is:
[0061]
[0062] After the alkylation reaction is completed, the carbonate solvent can be removed by distillation under reduced pressure, and then (Z)-2-(2-amino-4-thiazolyl)-2-triphenylmethoxy i...
Embodiment 1
[0072] A. Alkylation reaction
[0073] (1) Put 150Kg of dimethyl carbonate into a 500L reaction tank.
[0074] (2) Add 50kg (0.232kmol) of ethyl desmethylaminothioxamate and 80kg of dimethyl carbonate boron trifluoride complex (boron trifluoride content ≥ 40%).
[0075] (3) Start stirring and control the temperature at 20°C.
[0076] (4) in N 2 Under protection, 63.5 kg of triphenylmethanol was dropped in portions.
[0077] (5) After feeding the trityl alcohol, slowly raise the temperature to 35°C, and keep it warm for 13 hours. Sampling and analysis raw material residues below 1.0%.
[0078] (6) Turn on the distillation system, recover dimethyl carbonate under reduced pressure at 40°C, and cool down to 10°C after dimethyl carbonate is evaporated to dryness.
[0079](7) Add 200L of anhydrous methanol, an alcohol curing solvent, and stir for 2 hours until the product is completely cured.
[0080] (8) Put the material into a closed centrifuge to separate the product, wash...
Embodiment 2
[0106] A. Alkylation reaction
[0107] (1) Put 250kg of dimethyl carbonate into a 500L reaction tank.
[0108] (2) Add 50kg (0.2325kmol) of ethyl desmethylaminothioxamate and 80kg of dimethyl carbonate boron trifluoride complex (boron trifluoride content = 50%).
[0109] (3) Start stirring and control the temperature at 25°C.
[0110] (4) in N 2 Under protection, 63.5 kg of triphenylmethanol was dropped in portions.
[0111] (5) After feeding the trityl alcohol, slowly raise the temperature to 35°C, and keep it warm for 13 hours. Sampling and analysis raw material residues below 1.0%.
[0112] (6) Turn on the distillation system, recover dimethyl carbonate under reduced pressure at 40°C, and cool down to 10°C after dimethyl carbonate is evaporated to dryness.
[0113] (7) Add 200L of anhydrous ethanol, an alcohol curing solvent, and stir for 2 hours until the product is completely cured.
[0114] (8) Put the material into a closed centrifuge to separate the product, was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com