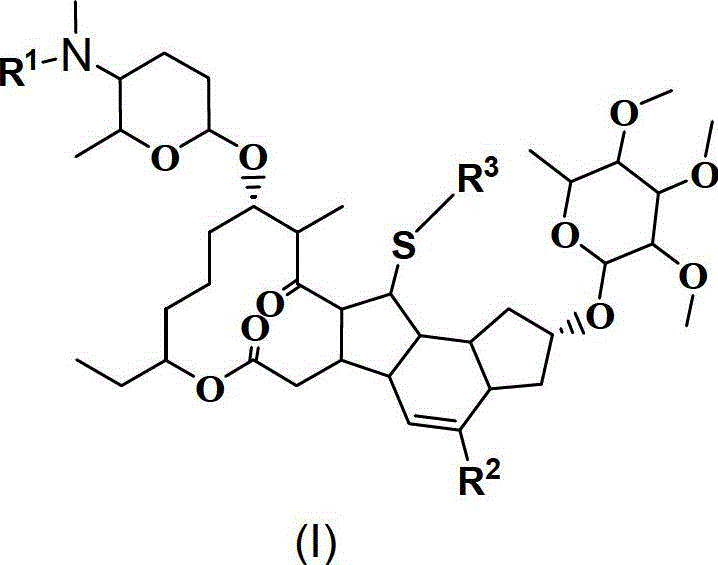
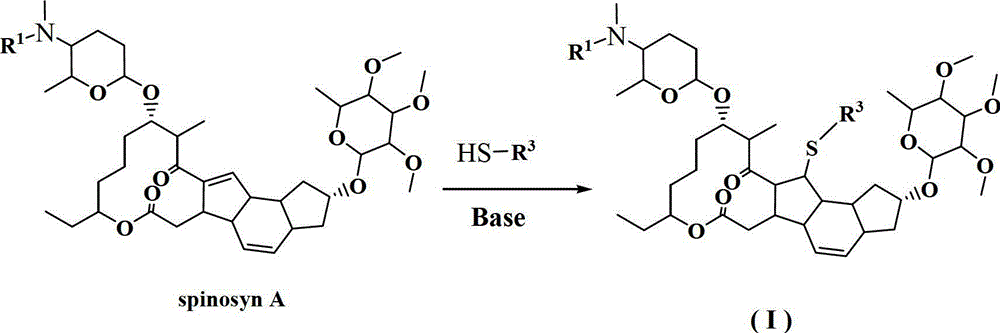
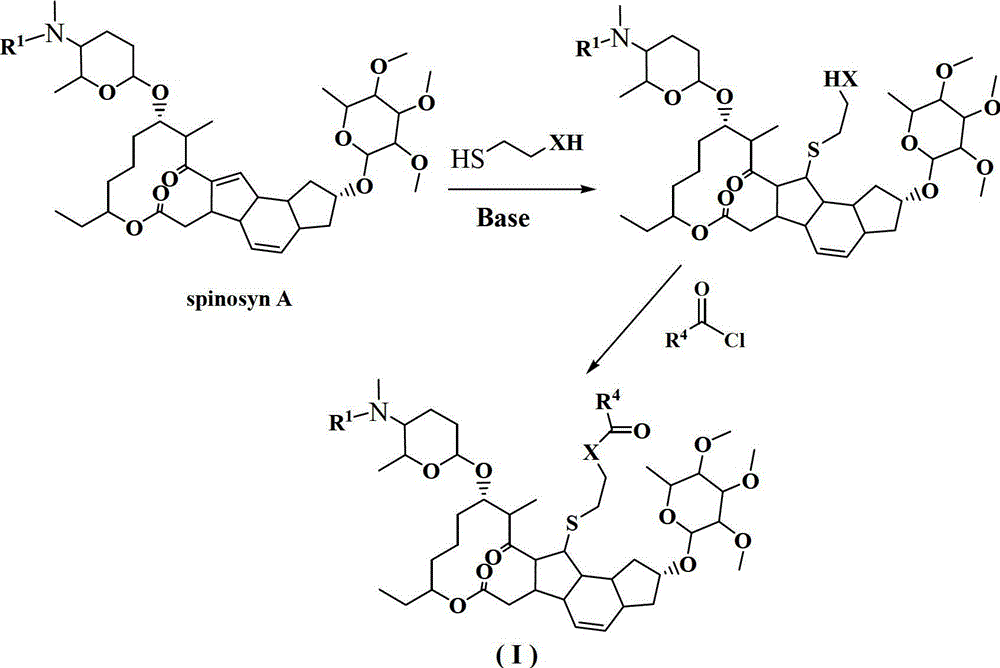
13-thioether substituted pleocidin derivative and preparation method thereof
A technology for spinosyn and derivatives, applied in the field of 13-thioether-substituted spinosyn derivatives and its preparation, can solve the problems of unreported insecticidal and acaricidal activities, and achieve high reaction yield and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0020] Example 1. Compound HNPC-X1001, i.e. 13-(2-hydroxyl) ethylthio-13,14-dihydro-spinosyn A synthesis
[0021]
[0022] Take 1 mL each of ether and acetone to make a mixed solution, place it in a 25 mL three-necked flask, add 100 mg of spinosyn A (0.136 mmol, 1.0 eq), and stir until completely dissolved. Add 50mg of anhydrous potassium carbonate (0.36mmol, 2.6eq) to the above solution at room temperature, and stir to form a white cloudy solution. Add 10 μl of mercaptoethanol to the above solution under nitrogen protection. Spinosad basically disappeared after 48 hours of reaction at room temperature, forming a large polar point (visible after iodine fumigation). Stop the reaction, spin off the solvent under reduced pressure, add 5 mL of saturated potassium carbonate aqueous solution, extract with 10 mL×3 ethyl acetate, combine the organic layers, dry over anhydrous sodium sulfate, and spin off the solvent under reduced pressure to obtain 70 mg of a white solid. After p...
example 2
[0023] Example 2. The compound HNPC-X1002 is the synthesis of 13-(2-acetoxy)ethylthio-13,14-dihydro-spinosyn A
[0024]
[0025] Take 100mg (0.124mmol, 1.0eq) of compound 1 into a 5mL dry single-necked bottle, add 3mL of anhydrous chloroform, and stir until completely dissolved. Add 80μl of triethylamine (0.496mmol, 4.0eq) to the above solution, stir well and then add 40μl of acetyl chloride (0.496mmol, 4.0eq). Transfer to an oil bath and heat to reflux. After the reaction was complete, cool to room temperature, add 2 mL of water, extract with 5 mL of chloroform x 3, combine the chloroform layers, dry and filter over anhydrous sodium sulfate, and spin off the solvent under reduced pressure to obtain a white solid. After column separation with ethyl acetate:methanol=20:1, 66 mg of white viscous solid was obtained, with a yield of 63%. 1 H NMR (400MHz, CDCl 3 ):δ5.88(d,1H),5.79(d,1H),4.85(s,1H),4.80(m,1H),4.40(d,1H),4.31(m,1H),4.10(m, 2H),3.73(m,2H),3.67(s,1H),3.40-3.60(m...
example 3
[0026] Example 3. Compound HNPC-X1003, namely the synthesis of 13-(2-benzoyloxy)ethylthio-13,14-dihydro-spinosyn A
[0027]
[0028] Take 50mg (0.062mmol, 1.0eq) of compound 1 into a 10mL dry single-necked bottle, add 3mL of anhydrous chloroform, and stir until completely dissolved. Add 17 μl of triethylamine (0.124 mmol, 2.0 eq) to the above solution, stir well and then add 15 μl of benzoyl chloride (0.124 mmol, 2.0 eq). Transfer to an oil bath and heat to reflux. After the reaction was complete, cool to room temperature, add 2 mL of water, extract with 5 mL of chloroform x 3, combine the chloroform layers, dry over anhydrous sodium sulfate, and spin off the solvent under reduced pressure to obtain a white viscous liquid. The column was passed through ethyl acetate to obtain 35 mg of a colorless viscous solid with a yield of 63%. 1 H NMR (400MHz, CDCl 3 ):δ8.06(d,J=7.2Hz,1H),7.54-7.60(m,1H),7.50(d,J=8.0Hz,2H),5.88(d,J=9.6Hz,1H),5.79 (d,J=9.6Hz,1H),4.77-4.90(m,2H),4.45-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com