Establishing method for drug screening model of high-throughput Japanese encephalitis virus helicase inhibitor
A Japanese encephalitis virus and helicase inhibitor technology, applied in the field of biomedicine, can solve the problem that the Japanese encephalitis virus helicase drug design model has not been established, and no Japanese encephalitis virus helicase drug report has been reported. and other problems, to achieve the effect of stable reaction, simple and easy method, and easy completion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Optimization of detection conditions for a drug screening model of a high-throughput Japanese encephalitis virus helicase inhibitor
[0047] (1) Preparation of helicase protein
[0048] The NS3 gene of Japanese encephalitis virus (Genbank accession number: U47032) was artificially synthesized, and the helicase fragment was digested with Nco I and Xho I, respectively, and the E. coli prokaryotic expression vector pET30a, T4 DNA Ligase (TakaRa, Dalian Bao Biological Engineering Co., Ltd. ) at 16°C overnight to obtain the recombinant plasmid pET30a-helicase, and the constructed recombinant plasmid pET30a-helicase was transformed into Escherichia coli BL21 (DE3) PlysS for expression. Collect the expressed protein supernatant with Ni 2+ Affinity purification, the purified helicase protein was identified by SDS-PAGE and Western blot, then glycerol was added to a final concentration of 20% (v / v), the concentration was determined by BCA method, and frozen at -80°C f...
Embodiment 2
[0069] Embodiment 2: the application of a drug screening model of a high-throughput Japanese encephalitis virus helicase inhibitor, its steps are as follows:
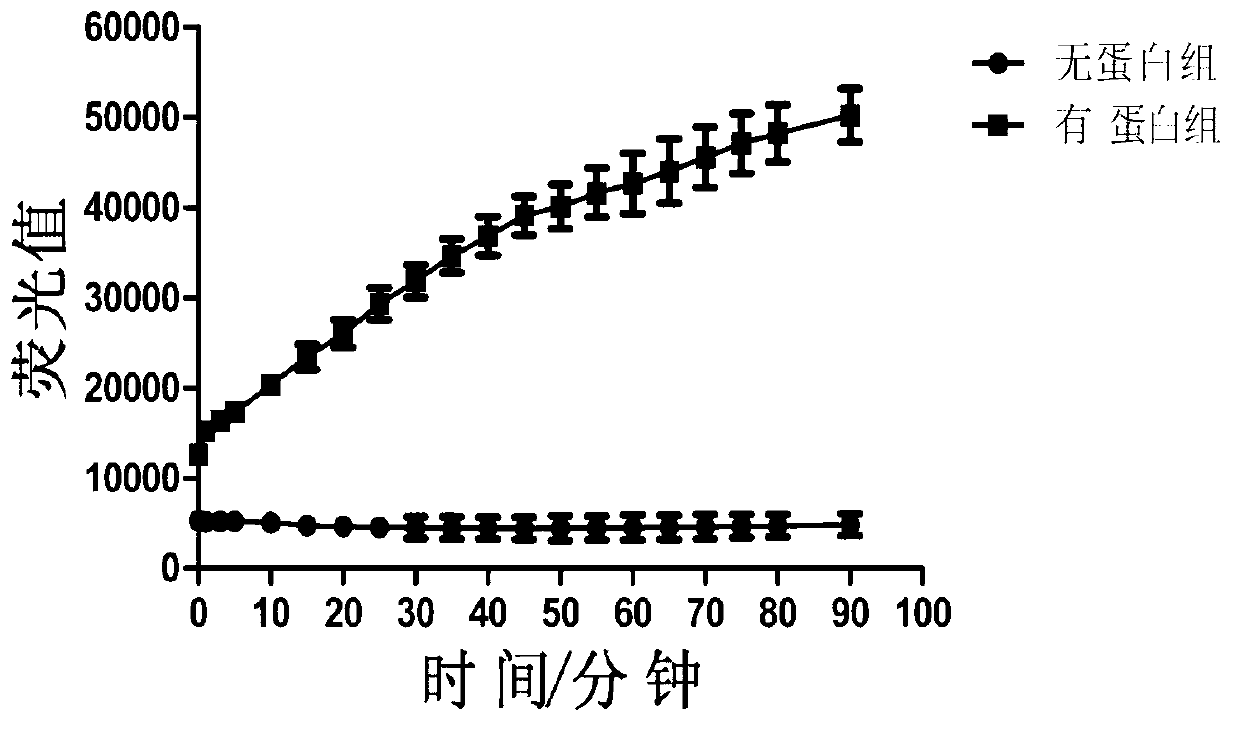
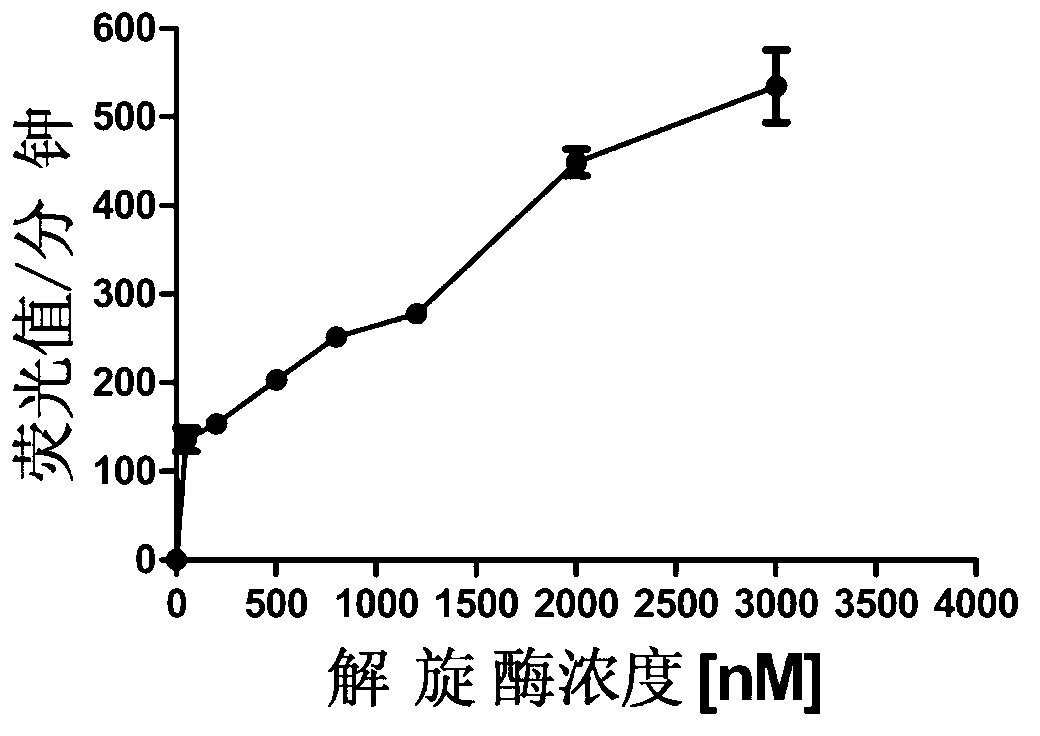
[0070] (1) Add 3uM of the helicase protein prepared in Example 1, the compound to be screened and the reaction buffer (buffer for dissolving protein, 20mM Tris-base, 150mM Tris-base, 150mM Nacl, 5% glycerol), mix and incubate for 5 minutes, add F chain 200nM and Q chain 240nM, incubate for another 5 minutes, add C chain 2.4uM and ATP 2mM, react at 37°C for 90 minutes, add reaction stop solution, in The fluorescence intensity in the system is detected in the fluorescence detector. At the same time, set up a control group without adding the compound to be screened (group C) and a control group without adding the substrate (group C1).
[0071] (2) Calculate the inhibition rate of the compound: inhibition rate = 1-(average fluorescence of experimental group-average fluorescence of group C1)÷(average fluorescence of group C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com