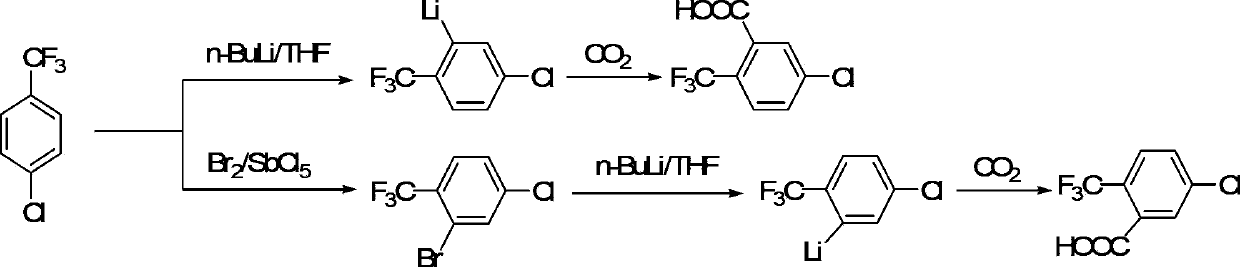
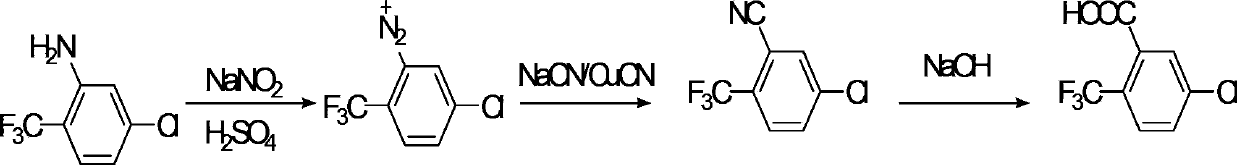
Preparation method of 2-chloro-5-(trifluoromethyl) benzoic acid
A technology of p-chlorobenzotrifluoride and trifluoromethyl, which is applied in the field of preparation of 2-chloro-5-benzoic acid, can solve the problems of low yield, long cycle, and increased cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
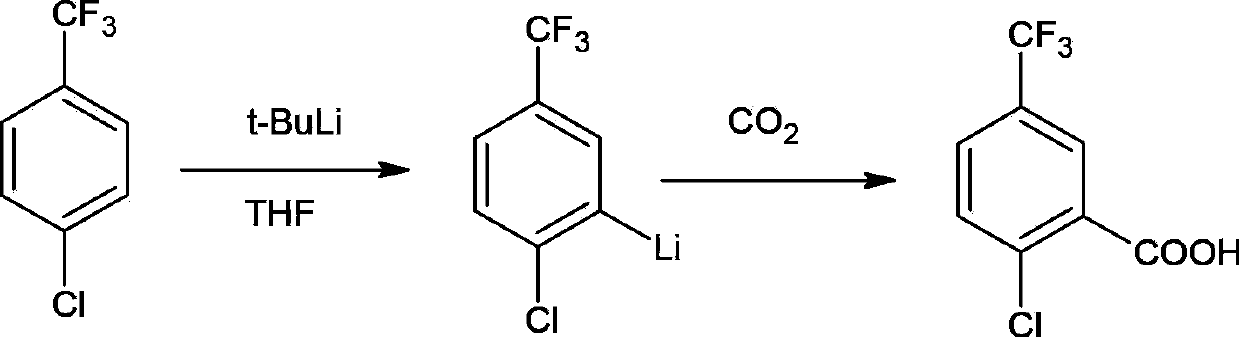
[0065] Taking p-chlorobenzotrifluoride as raw material to prepare 2-chloro-5-(trifluoromethyl)benzoic acid under the effect of tert-butyllithium / tetramethylethylenediamine, its chemical reaction equation is as follows:
[0066]
[0067] Weigh 18.1g (0.1mol) of the compound p-chlorotrifluoromethylbenzene and 11.6g (0.1mol) of tetramethylethylenediamine and dissolve it in 150ml of anhydrous tetrahydrofuran, and cool the reaction mixture to -75°C with a dry ice acetone bath The reaction system was vacuumed under nitrogen protection. Under the protection of nitrogen, extract 77.0ml (0.1mol) of tert-butyllithium (1.3M n-hexane solution) into a constant pressure dropping funnel, and then slowly add it to the reaction system. During the dropwise addition, the reaction temperature should not exceed - 70°C. After the dropwise addition, the reaction continued to stir for 1 hour, and the reaction solution was slowly poured on the surface of 50 g of dry ice, and continued to stir for ...
Embodiment 2
[0069] Using p-chlorobenzotrifluoride as raw material to prepare 2-chloro-5-(trifluoromethyl)benzoic acid under the action of tert-butyllithium / N,N-diisopropylethylamine, the chemical reaction equation is as follows:
[0070]
[0071] Weigh 18.1g (0.1mol) of the compound p-chlorotrifluoromethylbenzene and 12.9g (0.1mol) of N,N-diisopropylethylamine and dissolve it in 150ml of anhydrous tetrahydrofuran, and cool down the reaction mixture with a dry ice acetone bath To -75°C, the reaction system was evacuated under nitrogen protection. Under the protection of nitrogen, extract 77.0ml (0.1mol) of tert-butyllithium (1.3M n-hexane solution) into a constant pressure dropping funnel, and then slowly add it to the reaction system. During the dropwise addition, the reaction temperature should not exceed - 70°C. After the dropwise addition was completed, the reaction continued to stir for 1 hour, and the reaction solution was slowly poured on the surface of 50 g of dry ice, and cont...
Embodiment 3
[0073] A kind of preparation method of 2-chloro-5-(trifluoromethyl)benzoic acid, its chemical equation is identical with embodiment 1, specifically comprises the steps:
[0074] (1) Dissolve 0.08mol of p-chlorobenzotrifluoride and 0.1mol of tetramethylethylenediamine in 150mL of anhydrous tetrahydrofuran, cool down to -80°C, fill with nitrogen for protection, and then dropwise add 0.12mol of tert-butyl 88 mL of n-hexane solution of lithium lithium was stirred for reaction, and after the dropwise addition was completed, stirring was continued for 1.2 h to obtain the lithium salt of p-chlorotrifluorotoluene;
[0075] (2) Slowly pour the p-chlorobenzotrifluorotoluene lithium salt obtained in step (1) onto the surface of 50g of dry ice, stir the reaction solution for 60min, and after the dry ice has evaporated, add dropwise the concentration of 8mol / L at 15°C Hydrochloric acid aqueous solution 10mL, adjust the pH value to 3, distill the reaction liquid under reduced pressure, remo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| flash point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com