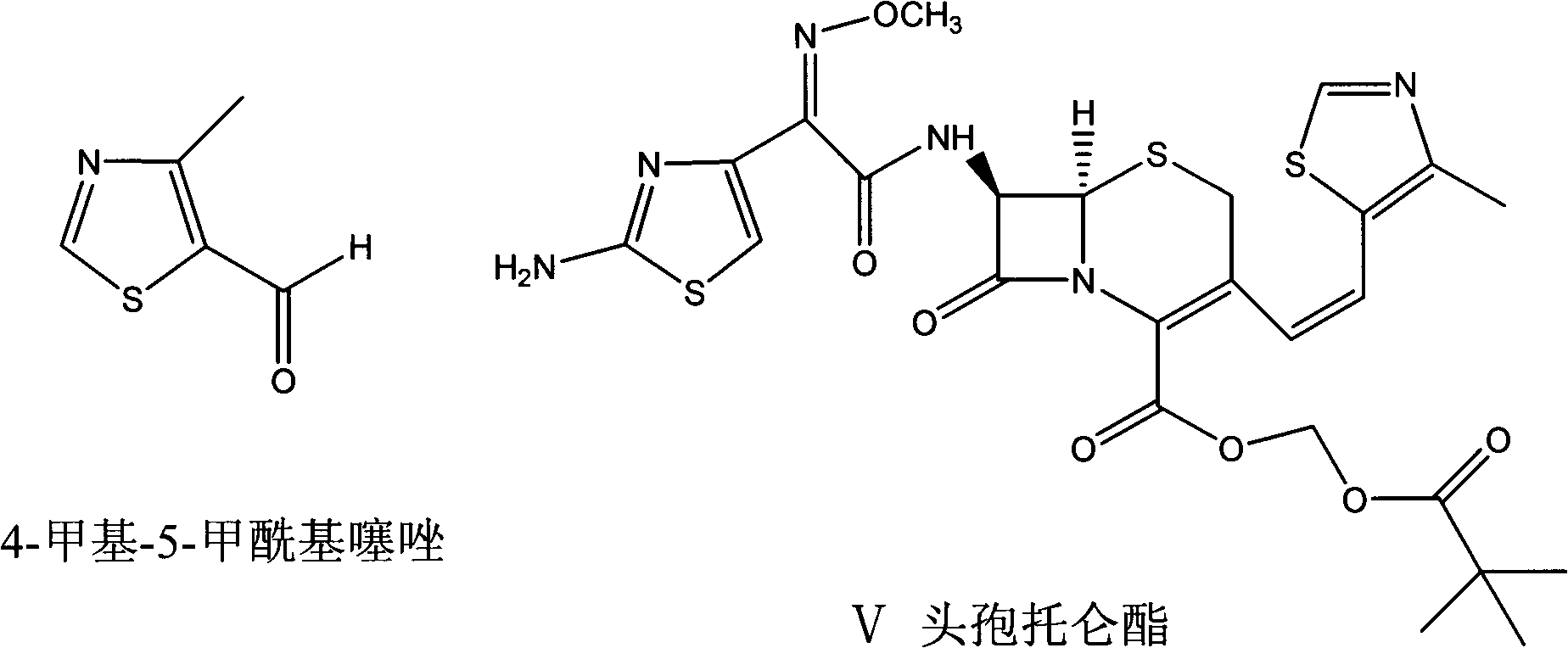
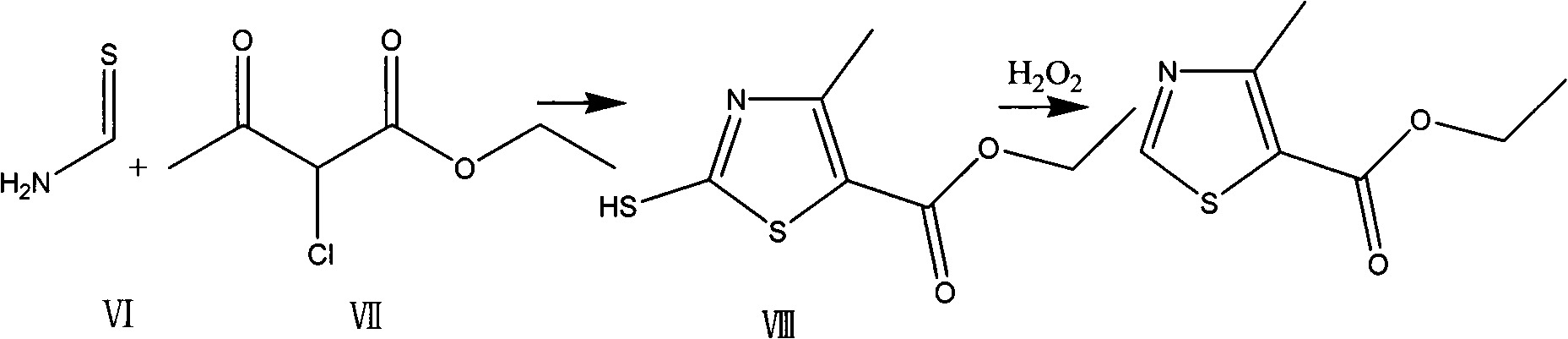
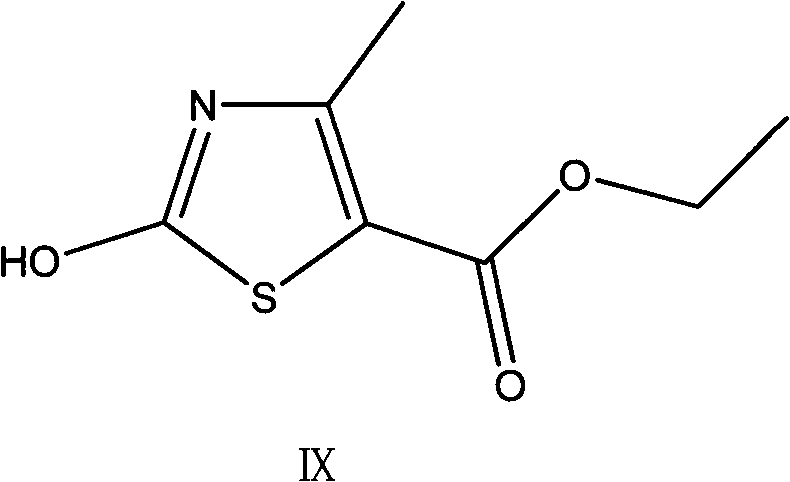
Preparation method of thiazole compounds
A compound and thiazole-based technology, applied in the field of preparation of thiazole-based compounds, can solve the problems of unfavorable industrial production and environmental protection, expensive raw materials, high price of finished products, etc., and achieve the advantages of industrial production and environmental protection, simple post-processing, solvent The effect of consumption saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation of tert-butyl nitrite
[0037] Add 38.0g (0.55mol) sodium nitrite and 150ml water in the 250ml three-necked reaction bottle that agitator and thermometer are housed, the reaction bottle is placed in ice-salt bath and stirred, the solution temperature is down to 0 ℃. Mix 10ml of water, 13.6ml (25.0g, 0.25mol) of 98% concentrated sulfuric acid (density 1.84g / ml), 45.7ml of tert-butanol (37.0g, 0.5mol) and pre-cool to 0°C, then slowly Add it dropwise to the above sodium nitrite solution, control the temperature at ±1°C, the whole dropping process takes 1-1.5h, and react for 1-2h after the dropping.
[0038] After the reaction was completed, the mixture was placed under an ice-salt bath and the layers were separated. The solution was poured into a separatory funnel to remove a small amount of solid sodium sulfate, and the upper light yellow organic layer was separated. The organic layer was washed twice with 100ml aqueous solution containing 2g sodium...
Embodiment 2
[0039] Embodiment 2 Preparation of n-butyl nitrite
[0040] Add 38g of sodium nitrite and 150ml of water into a 250ml three-necked bottle, dissolve and cool down to -5-0°C. Mix 10ml of water, 13.6ml (25.0g, 0.25mol) of 98% concentrated sulfuric acid (density 1.84g / ml) and 37.0g of n-butanol, pre-cool to 0°C, and then slowly add it dropwise to the above nitrous acid In sodium solution, keep the temperature at 0°C. After dropping, continue to stir in an ice bath for 1h. Then the reaction solution is poured into a 250ml separating funnel, layered, and the light yellow liquid organic phase of the upper floor is separated, and the organic phase is washed twice with 100ml of aqueous solution containing 2.0g sodium bicarbonate and 25.0g sodium chloride (each 50ml ), then the organic layer was separated, dried with anhydrous sodium sulfate, and filtered to obtain 45.1 g of light yellow liquid which was n-butyl nitrite, with a yield of 87.6%.
Embodiment 3
[0041] Example 3 Preparation of 4-methyl-5-ethoxyformylthiazole
[0042] Add 0.78g (7.5mmol) tert-butyl nitrite and 7ml of anhydrous N,N-dimethylformamide to a 50ml three-neck flask equipped with a stirrer, a thermometer and a reflux condenser with a sealed balloon, and heat up to 65°C under stirring. -70°C. Dissolve 0.93g (5.0mmol) of 2-amino-4-methyl-5-ethoxyformylthiazole in 3ml of anhydrous N,N-dimethylformamide, then slowly add it dropwise to the above solution, drop The addition process takes about 5 minutes, the temperature is controlled at 60-70°C, and the sealed balloon shows that there is gas generation in the reaction. After 30min, TLC showed that the starting material disappeared substantially (the developing solvent was cyclohexane:ethyl acetate 3:1, R f value is around 0.6). The reaction solution turned from light yellow to dark red. Lower the reaction solution to 0-5°C in an ice bath, add 50ml of water and 50ml of ethyl acetate under stirring, separate the u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com