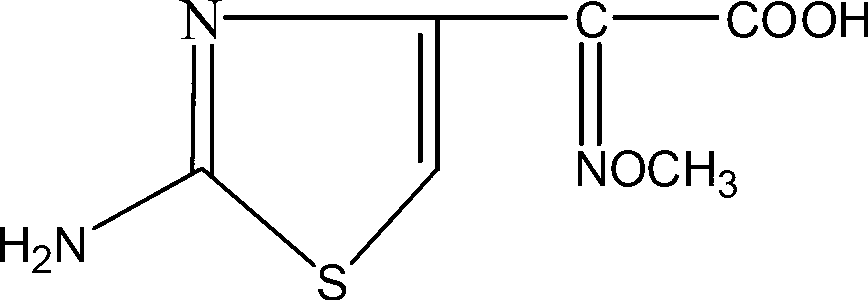
Synthetic method of aminothiazoly loximate
A technology of aminothiaxamic acid and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of long production cycle, excessive waste water, high production cost, etc., achieve the effects of reducing the discharge of three wastes, simplifying the operation process, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Add 60mL of ethanol aqueous solution (volume ratio 1:2), 20mL of ethyl acetoacetate, and 11g of ethyl nitrite into a three-necked flask, stir, and add 25g of glacial acetic acid dropwise at a temperature of 5±3°C. Continue to stir and react for 2h after completion; pass into N after completion of the reaction 2 1h, then add 0.4g tetrabutylammonium iodide, start to drop 20mL of dimethyl sulfate, control the temperature at 11±3°C, pH value is 8, react for 2h; dissolve 10g of triphosgene in 20mL of chloroform and add 0.5mL of DMF, Make a triphosgene solution, after the reaction is completed, slowly add the prepared triphosgene solution, control the reaction temperature at 15±3°C, and react for 2 hours; after the reaction is completed, add 10mL of ethanol aqueous solution, add 8g of thiourea, 16g of sodium acetate and 0.5g Tetrabutylammonium iodide, the reaction temperature is controlled at 30±3°C, the dropwise addition time is 1.5h, the dropwise temperature is kept for...
Embodiment 2
[0041] (1) Add 60mL of ethanol aqueous solution (volume ratio 1:2), 20mL of ethyl acetoacetate, and 11g of methyl nitrite into a three-necked flask, stir, and add 48g of glacial acetic acid dropwise at a temperature of 3±3°C. Continue to stir and react for 2h after completion; pass into N after completion of the reaction 2 1h, then add 0.6g of benzyltriethylammonium bromide, start to drop 20mL of dimethyl sulfate, control the temperature at 12±3℃, pH value is 9, react for 3h; dissolve 10g of triphosgene in 20mL of chloroform and add 0.5 Prepare triphosgene solution in mL of triethylamine. After the reaction is completed, slowly add the prepared triphosgene solution, control the reaction temperature at 16±3°C, and react for 2.5 hours; 16g sodium acetate and 0.7g benzyltriethylammonium bromide, control the reaction temperature at 27±3°C, add dropwise for 1.5h, keep the temperature for 2h after dropping, adjust the pH value to 7.4 with sodium carbonate solution, and cool down to ...
Embodiment 3
[0047] (1) Add 60mL ethanol aqueous solution (volume ratio 1:2), 20mL ethyl acetoacetate, and 13g n-propyl nitrite into a three-necked flask, stir at a temperature of 5±3°C, add 40g of glacial acetic acid dropwise, and finish adding in 1 hour. After the addition, continue to stir the reaction for 2h; after the reaction is completed, feed N 2 1h, then add 0.8g hexadecyltrimethylammonium bromide, start to drop 20mL of dimethyl sulfate, control the temperature at 9±3℃, pH value is 8~10, react for 4h; dissolve 10g triphosgene in 20mL Chloroform and add 0.5mL DMF to make a triphosgene solution. After the reaction is completed, slowly add the prepared triphosgene solution, control the reaction temperature at 14±3°C, and react for 2.5h; after the reaction is completed, add 10mL of ethanol aqueous solution and 8g of thiourea , 16g of sodium acetate and 0.9g of cetyltrimethylammonium bromide, control the reaction temperature at 33±3°C, dropwise adding time is 1.5h, keep warm for 2h aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com