Synthesis method of water-soluble squarylium indocyanine multifunctional cell fluorescent dye
A technology of indole squaraine and fluorescent dyes, which is applied in the field of synthesis of squaraine, can solve the problems of poor water solubility and wide application, and achieve the effects of easy fluorescence imaging, broad application prospects, and strong fluorescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Organic synthesis of indole squaraine dyes functionalized with primary amino groups.
[0032] 1) Add p-carboxyphenylhydrazine hydrochloride (5.66 g, 30 mmol) and 3-methyl-2-butanone (9.6 mL, 90 mmol) (molar ratio 1:3) into the reaction flask, and add solvent Glacial acetic acid 20 ml. The solution was heated to reflux temperature under nitrogen atmosphere and reacted for 12 hours to obtain 3H-indoline product.
[0033] 2) Add 3H-indoline (0.82 g, 4 mmol) and p-carboxybenzyl bromide (0.86 g, 4 mmol) (1:1 molar ratio) into the reaction flask, and add 10 ml of solvent acetonitrile. The solution was heated to reflux temperature under a nitrogen atmosphere, and reacted for 24 hours to obtain quaternized indoline derivatives.
[0034] 3) Add indoline derivatives (836 mg, 2 mmol) and squaraine (114 mg, 1 mmol) (molar ratio 1:0.5) into the reaction flask, and add 12 ml of solvent (n-butanol:toluene:pyridine =1:1:1), reflux reaction under nitrogen atmosphere for 24 h...
Embodiment 2
[0038] Example 2: Organic synthesis of indole squaraine dyes functionalized with tertiary amino groups.
[0039] 1) Implementation steps 1-3 are the same as implementation example 1.
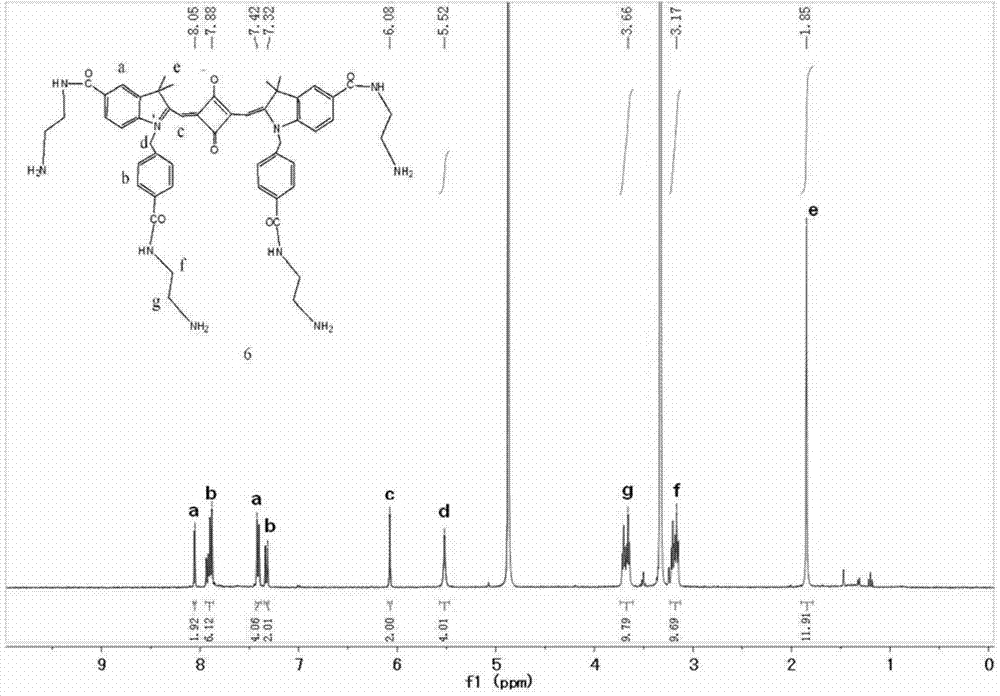
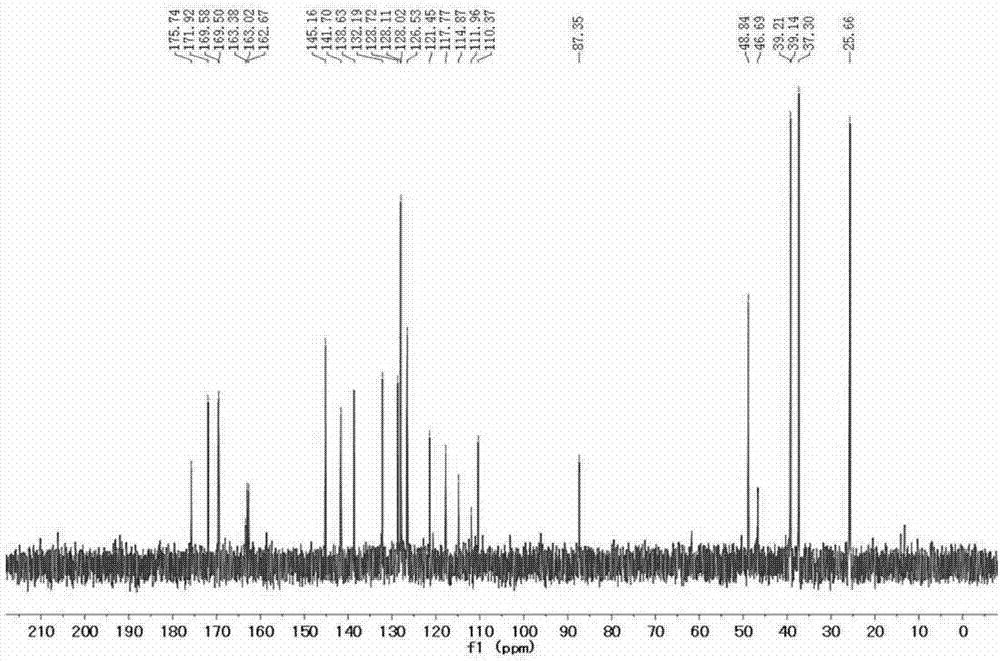
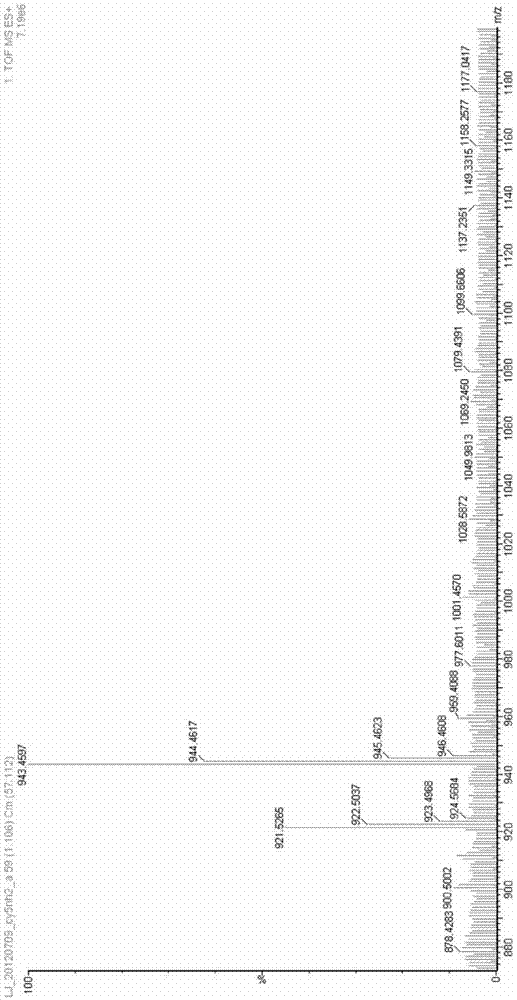
[0040] 4) Add carboxy-functionalized squaraine (301.2 mg, 0.4 mmol), HATU (1.52 g, 4 mmol) and DIPEA (1.32 mL, 8 mmol) (molar ratio 1:10:20) into the reaction flask, and Add solvent DMF 10 ml, stir at room temperature under nitrogen atmosphere for 10 minutes, then add N, N'-dimethylethylenediamine (282 mg, 3.2 mmol) and react for 0.5 hours to obtain tertiary amino functionalized indole squaraine dye. Its structural formula is as follows:
[0041]
Embodiment 3
[0042] Implementation Example 3: This type of dye specifically marks the cell membrane of living tissue cells.
[0043] The dyes were incubated with living cells of Drosophila and salivary gland tissues of transgenic Drosophila for 1 hour. The dyes could specifically label the cell membranes of the cells and overlap well with the cell membranes expressing green fluorescent protein.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com