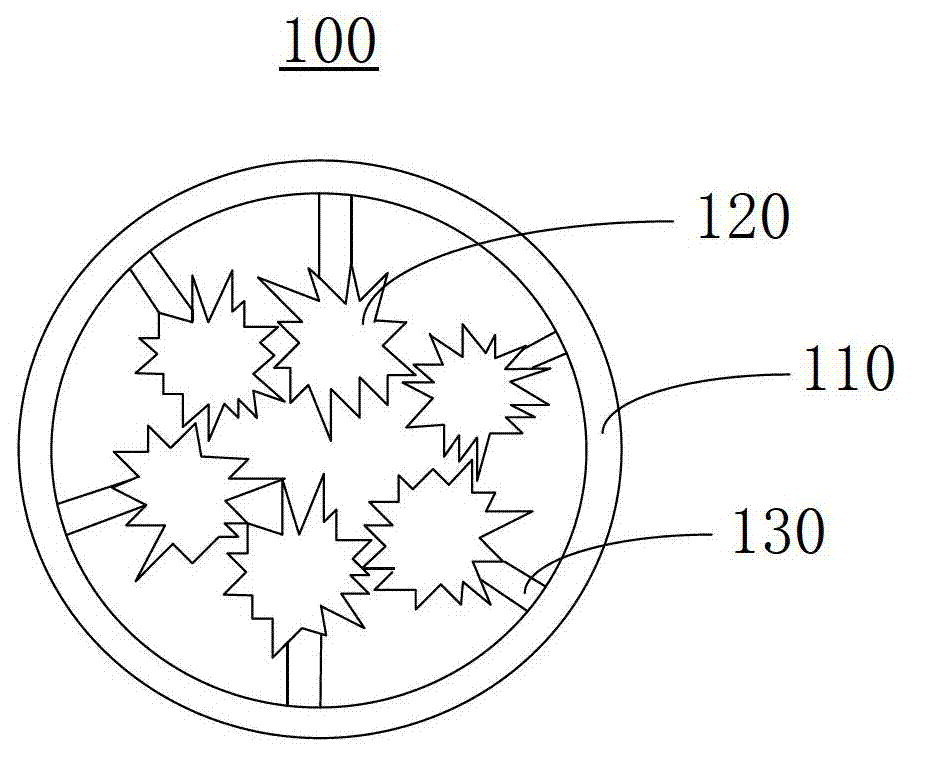
Diagnosis-treatment integrative medicine carrying polymer and preparation method thereof
A drug-loaded polymer, photosensitive drug technology, applied in the field of medicine, can solve problems such as poor compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
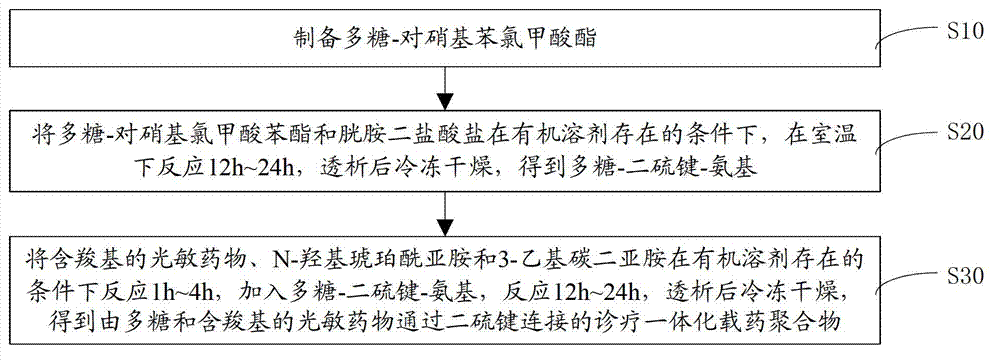
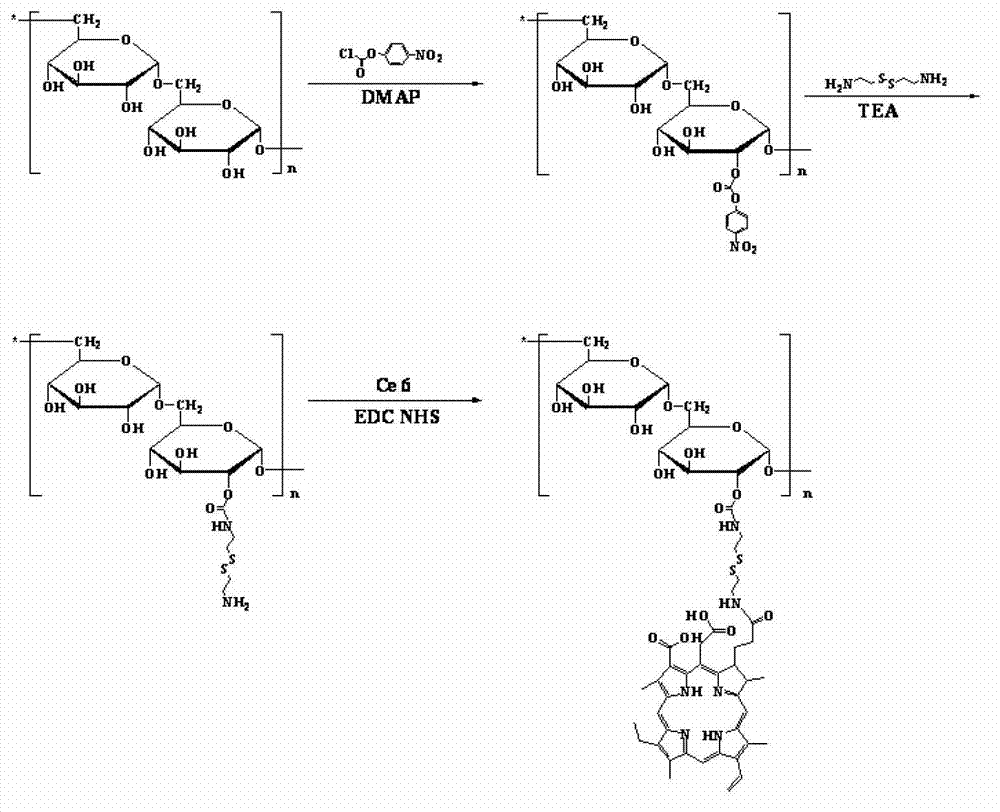
[0038] Please refer to figure 2 and image 3 A method for preparing a drug-loaded polymer integrated with diagnosis and treatment in one embodiment, comprising the following steps:
[0039] S10. Preparation of polysaccharide-phenyl p-nitrochloroformate. The concrete steps of preparing polysaccharide-p-nitrochloroformic acid phenyl ester are:
[0040] Dissolve polysaccharide, phenyl p-nitrochloroformate and 4-dimethylaminopyridine in a mixed solution of dimethyl sulfoxide and pyridine, react in an ice bath for 4h~6h, precipitate, centrifuge, and vacuum dry to obtain polysaccharide- Phenyl p-nitrochloroformate.
[0041]The polysaccharide may be at least one of dextran, sodium alginate, hyaluronic acid, heparin, chondroitin sulfate, pectin, pullulan and cyclodextrin. When the polysaccharide is at least one of dextran, sodium alginate, hyaluronic acid, heparin, chondroitin sulfate, pectin, pullulan and cyclodextrin, it has low price, good biocompatibility, and degradation an...
Embodiment 1
[0065] Dissolve 1g of dextran, 0.7g of phenyl p-nitrochloroformate and 0.05g of 4-dimethylaminopyridine in 40mL of a mixed solution of dimethyl sulfoxide and pyridine with a volume ratio of 1:1, and react in an ice bath After 4 hours, it was dropped into 400 mL of a mixture of ethanol and ether, wherein the volume ratio of ethanol and ether was 1:1 to form a precipitate, which was collected by centrifugation and dried in vacuo to obtain dextran-phenyl p-nitrochloroformate.
[0066] 0.5 g of dextran-p-nitrochloroformate and 2 g of cystamine dihydrochloride were dissolved in 50 mL of dimethyl sulfoxide, and reacted at room temperature for 12 h. In ultrapure aqueous solution, using a dialysis membrane with a molecular weight cut-off of 3500, dialyzed for 24 hours, and freeze-dried to obtain dextran-SS-NH 2 .
[0067] Dissolve 0.4g Ce6, 0.2g NHS and 0.4g EDC in 20mL DMSO, react for 1h, then add 2g dextran-SS-NH 2 , Reaction 12h. In ultrapure aqueous solution, using a dialysis m...
Embodiment 2
[0072] Dissolve 1 g of pullulan, 0.7 g of phenyl p-nitrochloroformate, and 0.05 g of 4-dimethylaminopyridine in 40 mL of a mixed solution of dimethyl sulfoxide and pyridine at a volume ratio of 1:1, and place in an ice bath After reacting for 6 hours, drop into the mixed solution of 400mL ethanol and diethyl ether, wherein, the volume ratio of ethanol and diethyl ether is 1:1, a precipitate is formed, the precipitate is collected by centrifugation, and vacuum-dried to obtain pullulan-p-nitrochloroformic acid benzene ester.
[0073] Dissolve 0.5g of pullulan-phenyl p-nitrochloroformate and 1.5g of cystamine dihydrochloride in 50mL of dimethyl sulfoxide, and react at room temperature for 20h. In ultrapure aqueous solution, using a dialysis membrane with a molecular weight cut-off of 3500, dialyzed for 72 hours, and freeze-dried to obtain Pullulan-SS-NH 2 .
[0074] Dissolve 0.5g PPIX, 0.5g NHS and 1g EDC in 20mL DMSO, react for 4h, then add 10g Pullulan-SS-NH 2 , Reaction 20h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com