Benzoxazine resin containing sulfonic group, and preparation method and application thereof
A technology containing sulfonic acid groups and benzoxazine, which is applied in the field of polymer materials, can solve the problems of poor acid and alkali resistance, high cost, and easy hydrolysis, and achieve good alcohol resistance, strong acid resistance, and high thermal stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1 Synthesis of sulfonic acid-containing benzoxazine monomers based on aniline and sodium p-hydroxybenzenesulfonate
[0085] Dissolve 3.74mL of formaldehyde solution with a mass concentration of 37%, 2.34mL of aniline in 20mL of dioxane, and stir for 0.5h at room temperature, add 4.9g of sodium phenol p-sulfonate, heat up to 90°C, and react for 4h . Post-processing: the solvent was removed by rotary evaporation, and the crude product was vacuum-dried at 60°C to obtain a light yellow powder with a yield of 81%.
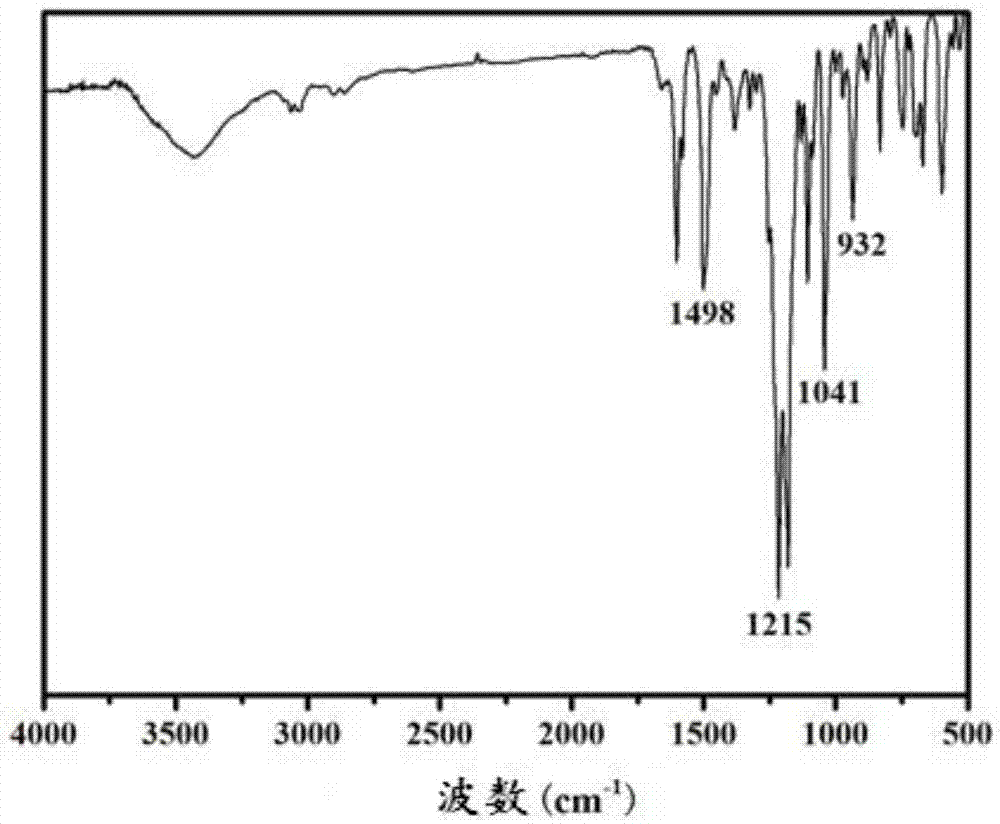
[0086] FTIR (KBr, cm -1 ):3331,3033(Ph-H),1600,1501(Ph),1233(stretch,C-O-C),932(oxazine ring).( figure 1 )
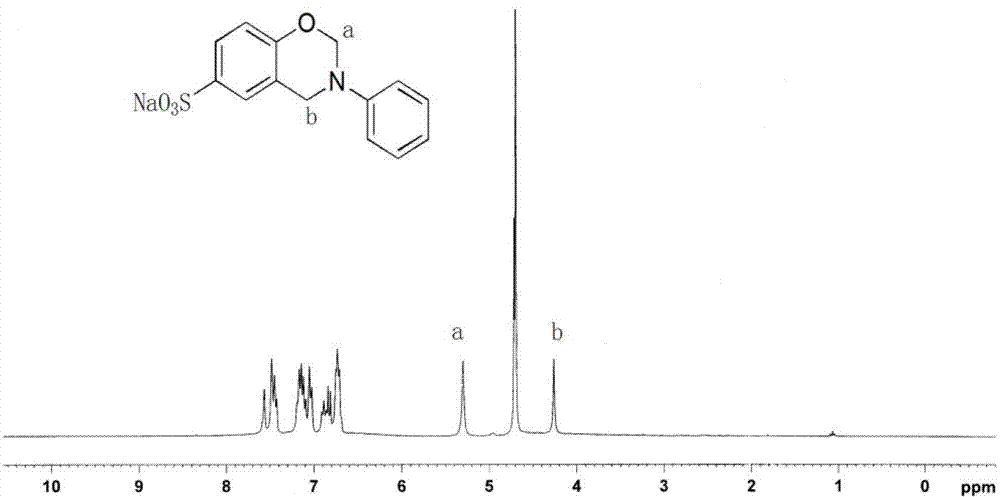
[0087] 1 H NMR (300MHz,H 2 O,ppm):4.28(s,2H,N-CH 2 -Ph),5.17(s,2H,N-CH 2 -O),6.74-7.68(m,8H,Ar-H).( figure 2 )
Embodiment 2
[0088] Example 2 Synthesis of sulfonic acid-containing benzoxazine monomers based on allylamine and sodium p-hydroxybenzenesulfonate
[0089] Add 1.0g of paraformaldehyde, 2.0mL of allylamine, 6.53g of sodium p-hydroxybenzenesulfonate, and 30mL of dioxane in sequence in a 100mL flask, stir and mix, gradually raise the temperature to reflux, and react at reflux temperature for 4h to obtain Orange yellow benzoxazine monomer solution, the reaction mixture was evaporated to remove the solvent, the obtained crude product was recrystallized with ethanol, and after drying, light yellow crystals were obtained with a yield of 81%.
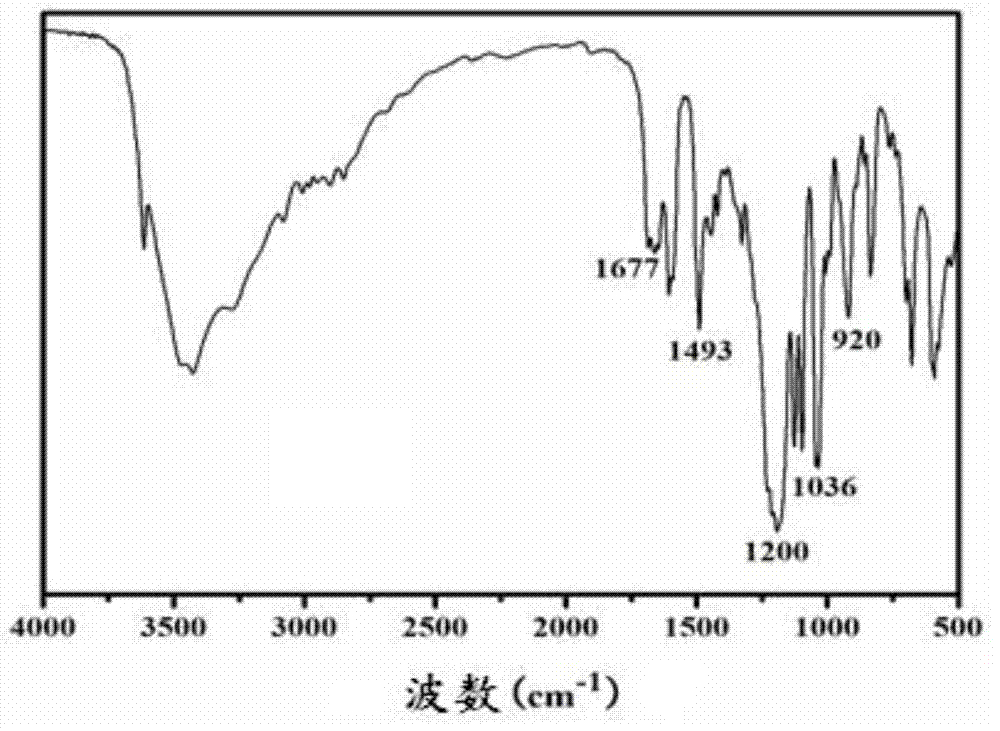
[0090] FTIR (KBr, cm -1 ):3331,3033(Ph-H),1193cm -1 (asymmetric C-O-C stretching), 1035cm -1 (symmetric C-O-C stretching),920cm -1 (oxazine ring). ( image 3 )
[0091] 1 H NMR (300MHz,H 2 O, ppm): 3.28 (s, 2H, -CH 2 -CH=CH 2 ),3.55(s,2H,N-CH 2 -Ph),4.35(s,2H,N-CH 2 -O),4.99(m,1H,-CH 2 -CH=CH 2 ),5.13(m,1H,-CH=CH 2 ),5.96(m,1H,-CH=CH 2 ),7.26...
Embodiment 3
[0092] Example 3 Synthesis of sulfonic acid-containing benzoxazine monomers based on phenol and sodium p-aminobenzenesulfonate
[0093]Add 0.75g of paraformaldehyde, 4.87g of sodium p-aminobenzenesulfonate, 2.35g of phenol, and 20mL of dioxane in a 100mL flask in sequence, and react at 90°C for 6h to obtain a pale yellow solution. After washing and recrystallization, a white solid was obtained with a yield of 85%.
[0094] FTIR (KBr, cm -1 ):3331,3033(Ph-H),1600,1501(Ph),1233(stretch,C-O-C),945(trisubstituted benzene ring).
[0095] 1 H NMR (300MHz,H 2 O,ppm):4.59(s,2H,N-CH 2 -Ph),5.20(s,2H,N-CH 2 -O),6.83-7.26(m,8H,Ar-H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| breaking strength | aaaaa | aaaaa |
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com