Preparation method of low-moisture and high-stability sterile cefuroxime sodium
A cefuroxime sodium and high stability technology, which is applied in the field of drug synthesis, can solve the problems of long drying time, unstable quality, and difficult to achieve, and achieve the effects of improving equipment production capacity, reducing production costs, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
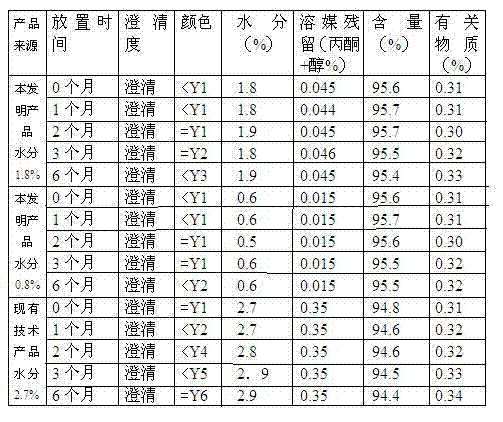
[0030] 1. Add 100Kg of cefuroxime acid, 900L of anhydrous acetone, and 800L of absolute ethanol into a 1500L dissolving tank, stir and dissolve at a temperature of 20 degrees, then add 10Kg of activated carbon, decolorize for 30 minutes, decarbonize, filter aseptically, and filter to no Bacteria crystallization tank.
[0031] 2. In the sodium-alkali solution decolorization tank, add 120L absolute ethanol, 40Kg sodium isooctanoate, and 5Kg activated carbon at a temperature of 20 degrees and stir for 20 minutes to decolorize. After decarburization and sterile filtration, filter the liquid into a sterile metering tank.
[0032] 3. Stir at a slow speed at a temperature of 28-30 degrees, and control the stirring speed at 20-40r / min. Add the sodium-alkali solution dropwise into the crystallization tank. After about 50 minutes, the crystallization precipitates, and then slowly add 300L sterile-filtered Anhydrous acetone, stirred for 30min.
[0033] 4. The crystallization is hydrauli...
Embodiment 2
[0036] 1. Add 100Kg of cefuroxime acid, 1000L of anhydrous acetone, and 300L of anhydrous methanol into a 1500L dissolving tank, stir and dissolve at a temperature of 20 degrees, then add 10Kg of activated carbon, decolorize for 30 minutes, decarbonize, filter aseptically, and filter to no Bacteria crystallization tank.
[0037] 2. Add 80L of anhydrous methanol, 25Kg of sodium acetate trihydrate, and 5Kg of activated carbon to the sodium-alkali solution decolorization tank at a temperature of 20 degrees and stir for 20 minutes to decolorize. After decarburization and sterile filtration, filter the liquid into a sterile metering tank.
[0038] 3. With temperature controlled at 23-25°C and stirring at a slow speed, add the sodium-alkali solution dropwise into the crystallization tank for about 20 minutes, and then crystallize, then slowly add 700L sterile-filtered anhydrous acetone and stir for 30 minutes.
[0039] 4. The crystallization is hydraulically filtered into a three-in...
Embodiment 3
[0042] 1. Add 100Kg of cefuroxime acid, 800L of anhydrous acetone, and 700L of absolute ethanol into a 1500L dissolving tank, stir and dissolve at a temperature of 20 degrees, then add 10Kg of activated carbon, decolorize for 30 minutes, and decarbonize and filter aseptically until the liquid pressure reaches zero. Bacteria crystallization tank.
[0043] 2. In the sodium-alkali solution decolorization tank, add 120L absolute ethanol, 35Kg sodium isooctanoate, 5Kg activated carbon at a temperature of 20°C and stir for 20 minutes to decolorize. After decarburization and sterile filtration, filter the liquid into a sterile metering tank.
[0044] 3. With temperature controlled at 25-30°C and stirring at a slow speed, add the sodium-alkali solution dropwise into the crystallization tank for about 30 minutes, and then crystallize, then slowly add 900L sterile-filtered anhydrous acetone and stir for 30 minutes.
[0045] 4. The crystallization is hydraulically filtered into a three-i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com