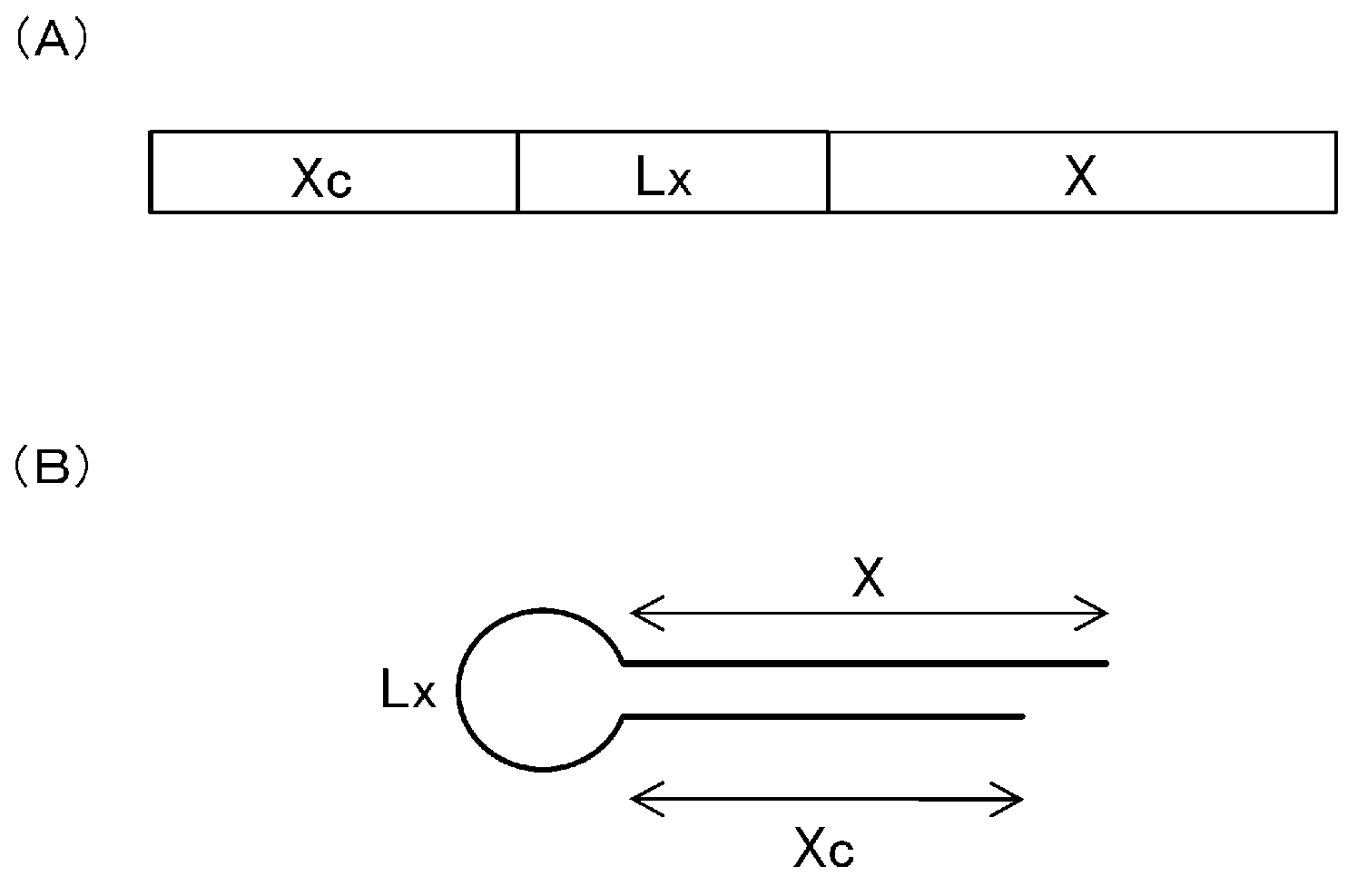
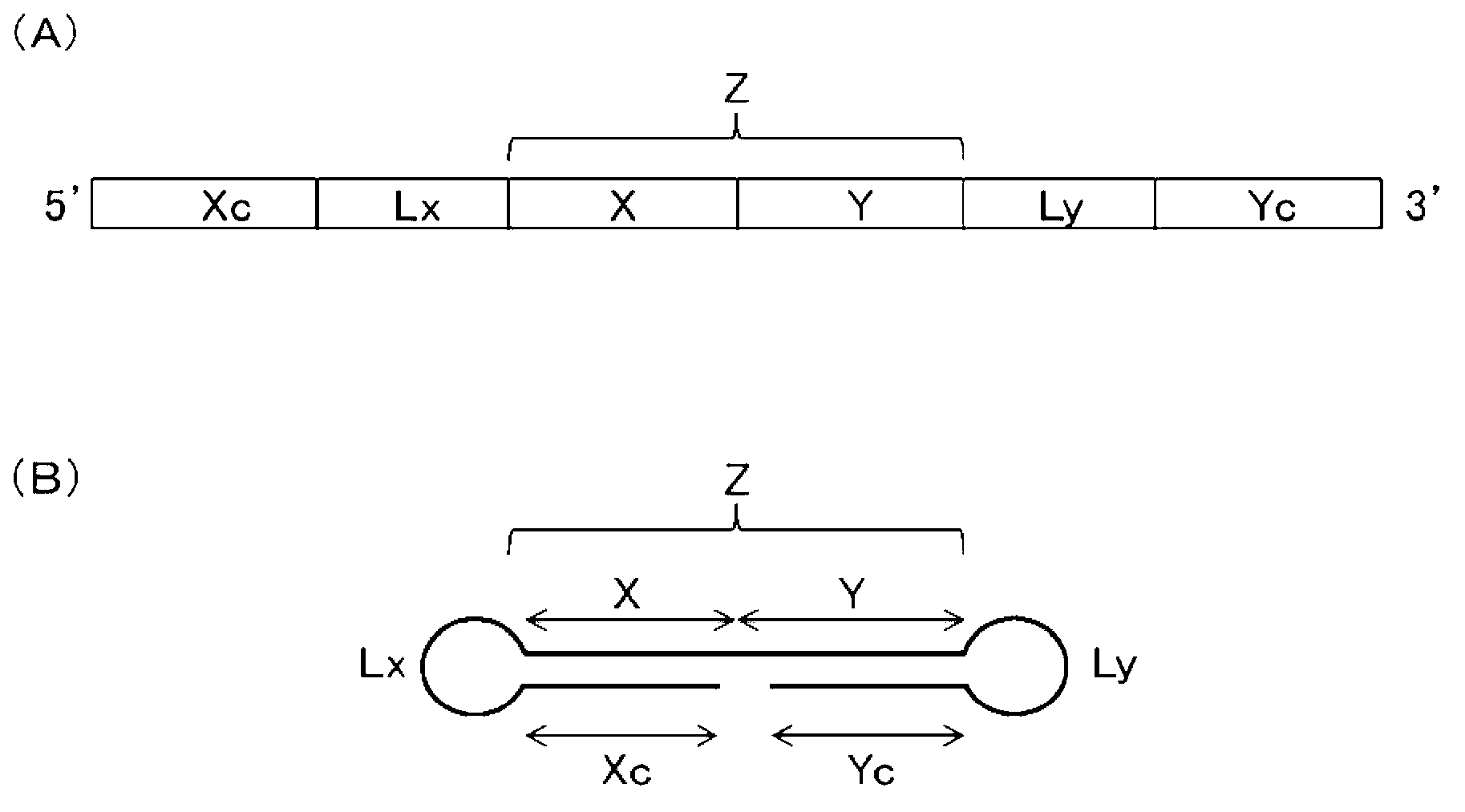
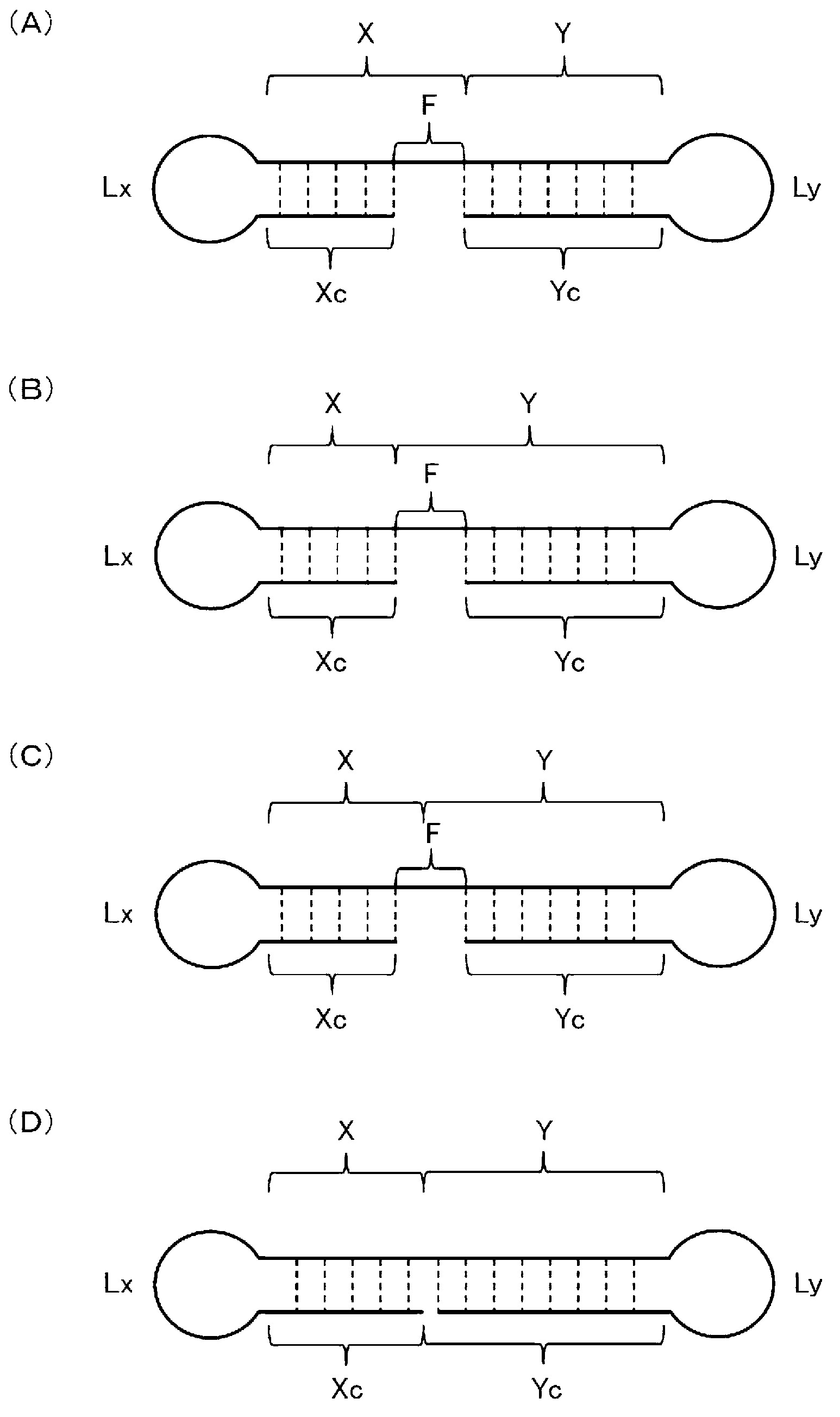
Single-stranded nucleic acid molecule having nitrogen-containing alicyclic skeleton
A single-stranded nucleic acid molecule and backbone technology, applied in the field of single-stranded nucleic acid molecules, can solve problems such as label changes, and achieve the effect of easy synthesis and efficient manufacturing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A1
[0367] 1. Synthesis of Prolinol
[0368] Prolinol protected with dimethoxytrityl was synthesized according to Scheme 1 shown below.
[0369] [Chemical formula 13]
[0370]
[0371] (1) Fmoc-L-prolinol (compound 2)
[0372] L-prolinol (compound 1) (0.61 g, 6.0 mmol) was dissolved in 70 mL of pure water to prepare an aqueous L-prolinol solution. N-(9-Fluorenemethoxycarbonyl)succinimide (Fmoc-OSu) (2.0 g, 6.0 mmol) was dissolved in 10 mL of THF. This THF solution was added to the above-mentioned L-prolinol aqueous solution, and the mixture was stirred for 1 hour to react both. The reaction solution was separated into a liquid fraction and a precipitate fraction, each fraction was extracted with ethyl acetate, and the organic layers were recovered separately. Then, after combining the respective organic layers, anhydrous sodium sulfate was added to absorb moisture (hereinafter referred to as drying). The above-mentioned organic layer was filtered, the filtrate was recovere...
Embodiment A2
[0405] Next, according to Scheme 3 shown in the following formula, an amidite derivative having L-proline was synthesized.
[0406] [Chemical formula 15]
[0407]
[0408] (1) DMTr-hydroxyamide amino-L-proline (compound 11)
[0409] In an ethanol solution (7 mL) containing DMTr-amide-L-proline (compound 6) (1.00 g, 2.05 mmol) and 5-hydroxyvaleraldehyde (0.33 g, 3.07 mmol), acetic acid was added under ice cooling buffer (7mL). After the mixture was stirred under ice-cooling for 20 minutes, sodium cyanoborohydride (0.77 g, 12.28 mmol) was added, and the mixture was further stirred at room temperature for 7 hours. The above mixed solution was diluted with dichloromethane, washed with water, and further washed with saturated brine. And the above-mentioned organic layer was recovered and dried over sodium sulfate. The above-mentioned organic layer was filtered, and the solvent was distilled off under reduced pressure with respect to the filtrate. The obtained residue was su...
Embodiment A3
[0433] (Example A3) Synthesis of proline diamide amidite
[0434] To generate nucleic acid molecules of the invention comprising linkers having a proline backbone, L-proline diamide amidite and D-proline diamide amidite were synthesized by Scheme 3 above.
[0435] (B3-1) L-proline diamide amidite
[0436] (1) Fmoc-hydroxyamide-L-proline (compound 4)
[0437] Compound 2 (Fmoc-L-proline) of Scheme 3 above was used as the starting material. The above compound 2 (10.00 g, 29.64 mmol), 4-amino-1-butanol (3.18 g, 35.56 mmol) and 1-hydroxybenzotriazole (10.90 g, 70.72 mmol) were mixed, and the above mixture was added under reduced pressure. Under degassing, filled with argon. Anhydrous acetonitrile (140 mL) was added to the above mixture at room temperature, followed by addition of an anhydrous acetonitrile solution (70 mL) of dicyclohexylcarbodiimide (7.34 g, 35.56 mmol), followed by stirring at room temperature for 15 minutes under an argon atmosphere. Hour. After completion o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com