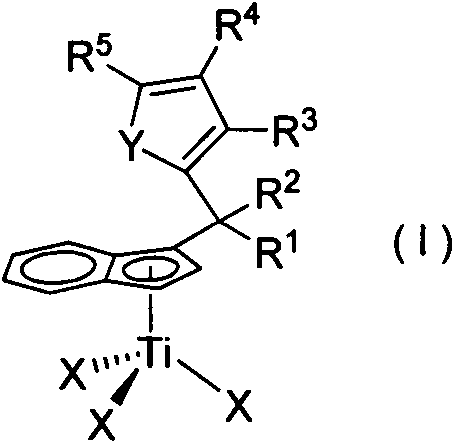
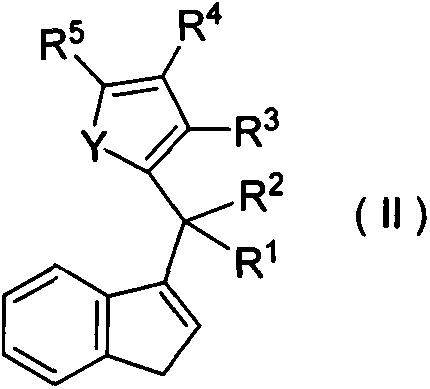
Preparation method and application of high-selectivity ethylene trimerization catalyst
A high-selectivity, ethylene trimerization technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., to reduce industrial costs, good temperature resistance, good The effect of the catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of Substituted Indene Titanium Complex C1
[0036] (1) Preparation of Ligand Compound L1
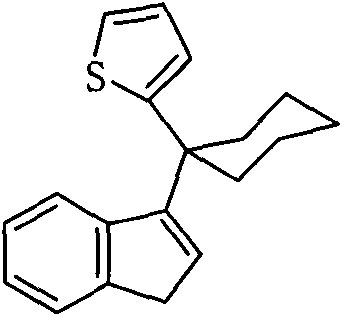
[0037] At ~78°C, add 7.8g (40mmol) 6,6-pentylidene benzofulvene dropwise to 40mL ether solution of 2-thiophene lithium salt (40mmol), react at room temperature for 1 day, hydrolyze, and use 50mL petroleum The organic layer was extracted with ether, and purified by column chromatography (petroleum ether as a developing solvent) to obtain 2.23 g of a light yellow solid with a yield of 20%. The structural formula is as follows:
[0038]
[0039] 1 H NMR (δ, ppm, CDCl 3 ): 7.43-7.41 (m, 1H, Ind-H), 7.24-7.22 (m, 1H, Ind-H), 7.11-7.08 (m, 3H, thienyl-H, Ind-H), 6.89-6.87 (m , 2H, thienyl-H, Ind-H), 6.42(t, J=2.0Hz, 1H, Ind-H), 3.38(d, J=2.0Hz, 2H, Ind-H), 2.50-2.42(m, 2H, (CH 2 ) 5 ), 2.25-2.17 (m, 2H, (CH 2 ) 5 ), 1.70-1.60 (m, 4H, (CH 2 ) 5 ), 1.57-1.47 (m, 2H, (CH 2 ) 5 )
[0040] (2) Preparation of complex C1
[0041] Add n-BuLi (6 mmol) dropwise to li...
Embodiment 2
[0045] Preparation of Substituted Indene Titanium Complex C2
[0046] (1) Preparation of Ligand Compound L2
[0047] At 78°C, add 9.4g (48mmol) of 6,6-pentylidene benzofulvene dropwise to 40mL of ether solution of 5-methyl-2-thiophene lithium salt (48mmol), react at room temperature for 1 day, and hydrolyze , the organic layer was extracted with 50 mL of petroleum ether, and purified by column chromatography (petroleum ether was used as a developing solvent) to obtain 4.2 g of a light yellow solid with a yield of 30%. The structural formula is as follows:
[0048]
[0049] 1 H NMR (400MHz, CDCl 3 ): δ7.47-7.41(m, 1H, Ind-H), 7.36-7.31(m, 1H, Ind-H), 7.16-7.01(m, 2H, Ind-H), 6.72-6.68(m, 1H , thienyl-H), 6.54(s, 1H, thienyl-H), 6.42(s, 1H, Ind-H), 3.39(s, 2H, Ind-H), 2.47-2.37(m, 5H, 2(CH 2 ) 5 , thienyl-CH 3 ), 2.27-2.10 (m, 2H, (CH 2 ) 5 ), 1.75-1.46 (m, 6H, (CH 2 ) 5 ).
[0050] (2) Preparation of complex C2
[0051] Add n-BuLi (6mmol) dropwise to ligand L3 (...
Embodiment 3
[0055] Preparation of Substituted Indene Titanium Complex C3
[0056] (1) Preparation of Ligand Compound L3
[0057] At 78°C, add 9.4g (48mmol) of 6,6-pentylidene benzofulvene dropwise to 40mL of ether solution of 3-methyl-2-thiophene lithium salt (48mmol), react at room temperature for 1 day, and hydrolyze , the organic layer was extracted with 50 mL of petroleum ether, and purified by column chromatography (petroleum ether was used as a developing solvent) to obtain 2.4 g of a light yellow solid with a yield of 17%. The structural formula is as follows:
[0058]
[0059] 1 H NMR (400MHz, CDCl 3 ): δ7.43 (d, J=7.2, 1H, Ind-H), 7.14-7.01 (m, 4H, 3Ind-H, thienyl-H), 6.69-6.61 (m, 1H, thienyl-H), 6.55 -6.38(m, 1H, Ind-H), 3.41(s, 2H, Ind-H), 2.56-2.45(m, 2H, (CH 2 ) 5 ), 2.21-2.11 (m, 2H, (CH 2 ) 5 ), 1.92-1.80 (m, 3H, thienyl-CH 3 ), 1.75-1.56 (m, 5H, (CH 2 ) 5 ), 1.51-1.41 (m, 1H, (CH2 ) 5 ).
[0060] (2) Preparation of complex C3
[0061] Add n-BuLi (6mmol) d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com