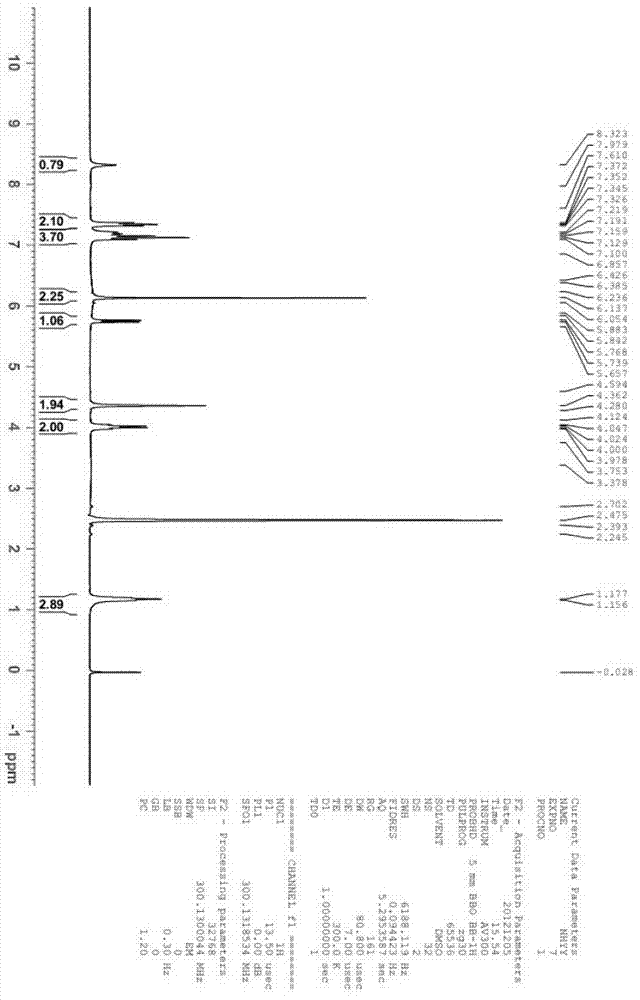
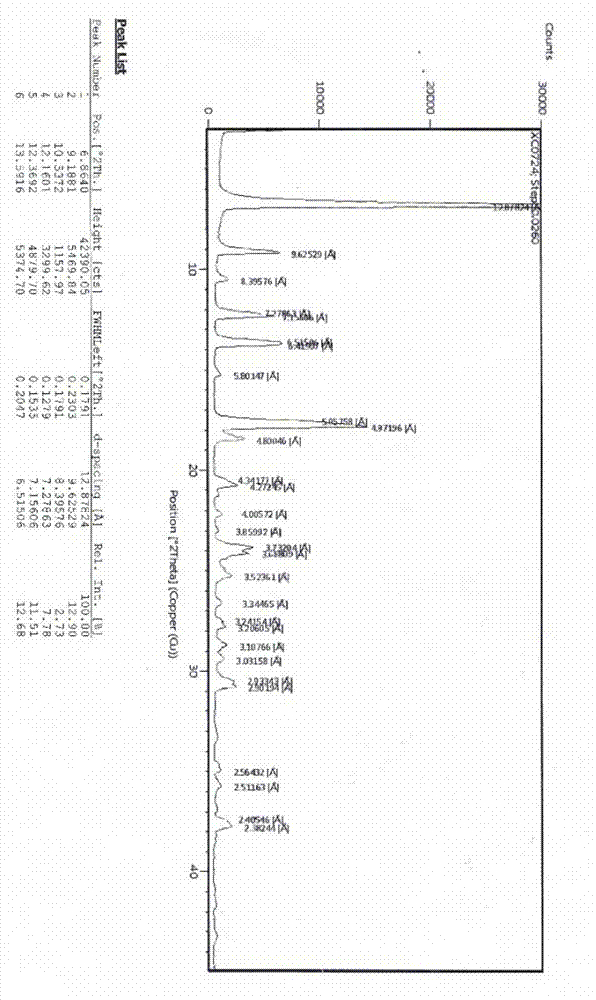
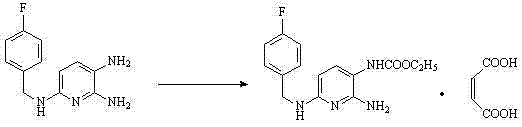
Synthetic method of flupirtine maleate A-type crystal compound and midbody
A technology of flupirtine maleate and an intermediate, which is applied in the field of synthesis of flupirtine maleate A crystal form compounds, can solve the problem of inability to obtain high-purity A crystal form flupirtine maleate samples and the purification effect of impurities problems such as poor solubility and low solubility, to achieve the effect of reducing the types of residual solvents, reducing environmental pollution and reducing difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Add 100g of 2-amino-3-nitro-6-chloropyridine, 87.5g of triethylamine, 20g of pyridine and 400ml of ethanol into a three-necked flask, heat to reflux under stirring, slowly add 80g of p-fluorobenzylamine dropwise, and react 3 After ~4 hours, after the reaction was completed, 500ml of purified water was added dropwise, slowly cooled to room temperature with stirring, filtered, and dried to obtain 2-amino-3-nitro-6-p-fluorobenzylaminopyridine.
[0062] Dissolve 41g of ferric chloride hexahydrate in 500ml of purified water, add 77.5g of activated carbon, heat to 50°C, add 30g of saturated sodium hydroxide solution (16g of sodium hydroxide dissolved in 14ml of water), stir at 60°C for 1 hour, and drop to room temperature, filtered, and dried to obtain ferric hydroxide / activated carbon catalyst.
[0063] Add 104.8g of 2-amino-3-nitro-6-p-fluorobenzylaminopyridine, 54.4g of ferric hydroxide / activated carbon catalyst into a 2L reaction flask, add 1600ml of 95% ethanol, and heat...
Embodiment 2
[0067] Add 98g of 2-amino-3-nitro-6-chloropyridine, 63.7g of triethylamine, 5g of pyridine and 200ml of ethanol into a three-necked flask, heat to reflux under stirring, slowly add 80g of p-fluorobenzylamine dropwise, and react 5 hours, after the reaction was completed, 500ml of purified water was added dropwise, slowly cooled to room temperature with stirring, filtered, and dried to obtain 2-amino-3-nitro-6-p-fluorobenzylaminopyridine.
[0068] Dissolve 41g of ferric chloride hexahydrate in 1000ml of purified water, add 300g of activated carbon, heat to 50°C, add 90g of saturated sodium hydroxide solution (48g of sodium hydroxide dissolved in 42ml of water), stir at 60°C for 1 hour, and cool to room temperature , filtered, and dried to obtain ferric hydroxide / activated carbon catalyst.
[0069] Add 104.8g of 2-amino-3-nitro-6-p-fluorobenzylaminopyridine, 80g of ferric hydroxide / activated carbon catalyst into a 2L reaction flask, add 2000ml of 95% ethanol, and heat to 70~90°C ...
Embodiment 3
[0073] Add 246g of 2-amino-3-nitro-6-chloropyridine, 254g of triethylamine and 800ml of ethanol into a three-necked flask, heat to reflux under stirring, slowly add 80g of p-fluorobenzylamine dropwise, react for 6 hours, and the reaction is complete Afterwards, 500 ml of purified water was added dropwise, slowly cooled to room temperature with stirring, filtered, and dried to obtain 2-amino-3-nitro-6-p-fluorobenzylaminopyridine.
[0074] Dissolve 41g of ferric chloride hexahydrate in 200ml of purified water, add 20g of activated carbon, heat to 50°C, add 45g of saturated sodium hydroxide solution (24g of sodium hydroxide dissolved in 21ml of water), stir at 60°C for 1 hour, and cool to room temperature , filtered, and dried to obtain ferric hydroxide / activated carbon catalyst.
[0075] Add 104.8g of 2-amino-3-nitro-6-p-fluorobenzylaminopyridine, 20g of ferric hydroxide / activated carbon catalyst into a 2L reaction flask, add 1200ml of 95% ethanol, and heat to 90°C under stirrin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com