Acrylate functional polymer with photo-initiation active end group and preparation method of polymer
A technology of active end groups and acrylates, applied in polyurea/polyurethane coatings, coatings, etc., can solve the problem of low end group concentration, insignificant effect of end group chemical reactions on polymers, and no chain reaction effect, etc. problem, to achieve the effect of inhibiting migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
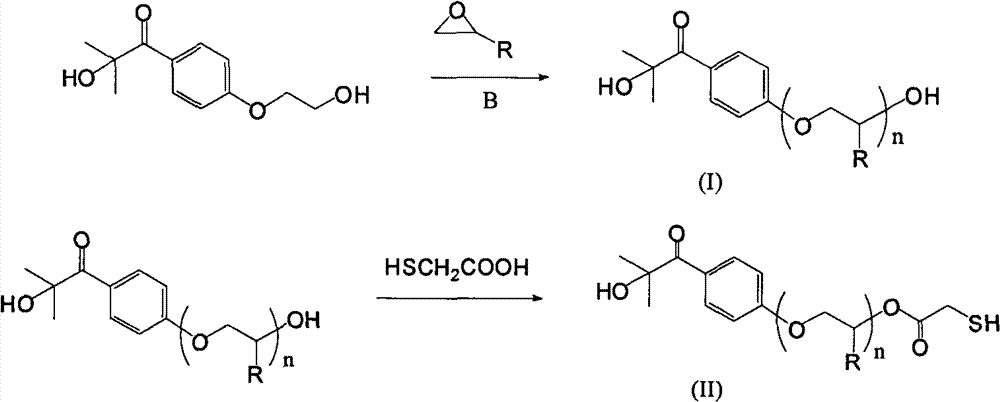
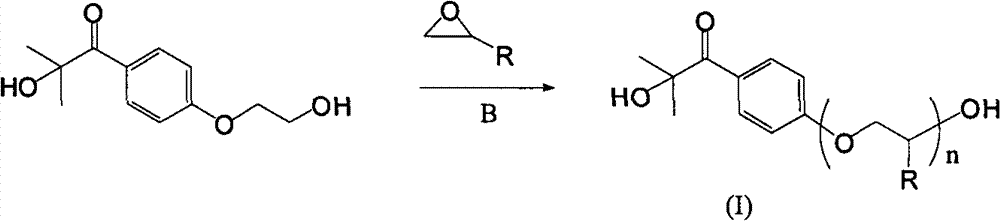
[0047] In a 500ml airtight three-neck bottle, add 0.1mol of vacuum-dried photoinitiator Darocur 2959 and sodium hydride mineral oil solution containing 0.15mol of active ingredients in 250ml of pure toluene solvent. Ethylene oxide was reacted at room temperature for 3 hours and the pressure in the reactor did not change. Stop responding. The composition of Darocur 2959 is 2-hydroxy-4'-(2-hydroxyethoxy)-2-methylpropiophenone.
[0048] The above-mentioned pure toluene can be prepared by using crude toluene to remove water and dry with calcium hydride, and then distill and purify.
[0049] Then the extraction is washed and extracted with dilute sodium chloride brine with a concentration of 5% by weight to remove by-products and impurities, and the solvent is distilled off to obtain a polyether chain modified photoinitiator. The average degree of polymerization of the oligoether chain is 3.3 as determined by proton nuclear magnetic spectrum. The structure of the oligoether is s...
Embodiment 2
[0057] In the airtight three-neck bottle of 500ml volume, in the 250ml toluene solvent that sodium hydride removes water and redistills, add 0.1mol vacuum-dried photoinitiator Darocur 2959 and the sodium hydride mineral oil solution that contains 0.15mol active ingredient, through steel bottle and Pipeline transportation, feed 1.0mol propylene oxide gas, and react at room temperature for 4 hours.
[0058] Then the extraction is washed and extracted with dilute sodium chloride brine with a concentration of 5% by weight to remove by-products and impurities, and the solvent is distilled off to obtain a polyether chain modified photoinitiator. The average degree of polymerization of polyoxypropylene is 8.1 as determined by H NMR spectroscopy, and the schematic diagram of the structure is shown in V. There may be isomerism at the methyl position in the propyleneoxy group.
[0059]
[0060] Take the photoinitiator modified by the polyether chain obtained above, dissolve it in 20...
Embodiment 3
[0066] In a closed three-necked bottle with a volume of 500ml, add 0.1mol of photoinitiator Darocur 2959 and sodium hydride mineral oil solution containing 0.15mol of active ingredients in 250ml of toluene solvent that has been dewatered and redistilled with sodium hydride, and transported through steel cylinders and pipelines. Pass through 0.68mol ethylene oxide, and react at room temperature for 3 hours. Dilute brine was washed and extracted to remove by-products and impurities, and the solvent was distilled off to obtain a photoinitiator modified with polyether chains. The average degree of polymerization of the polyether chains was determined by H NMR spectroscopy to be 5.7, and the structure was shown in VII.
[0067]
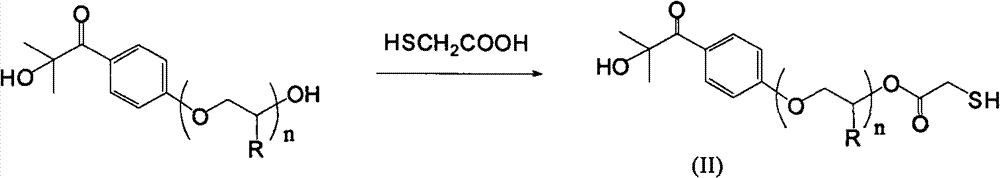
[0068] Get all the polyether chain-modified photoinitiators obtained above, dissolve in 200ml toluene, use 2mmol p-toluenesulfonic acid-N,N-dimethylbenzyl quaternary ammonium salt as catalyst, add 0.13mol thioglycolic acid, nitrogen Under protection, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com