Oxidation resistive amylase mutant as well as preparation method and application thereof
A technology of oxidation resistance and amylase, applied in biochemical equipment and methods, botany equipment and methods, applications, etc., can solve the problems of difficult to obtain target strains, blindness, etc., and achieve shortened transformation time and efficient degradation , Strong oxidation resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Analysis and method of site-directed mutation of amylase oxidation resistance
[0019] By analyzing the 3D spatial structure of amylase, the Met residues (M135, M204, M219, M237) that are not resistant to oxidation in the catalytic region were determined. At the same time, the analysis of other Met residues on the surface of the enzyme structure molecule around the active site found that M307 is on the surface of the amylase molecule and is easily oxidized by oxidants, resulting in reduced or inactivated amylase activity.
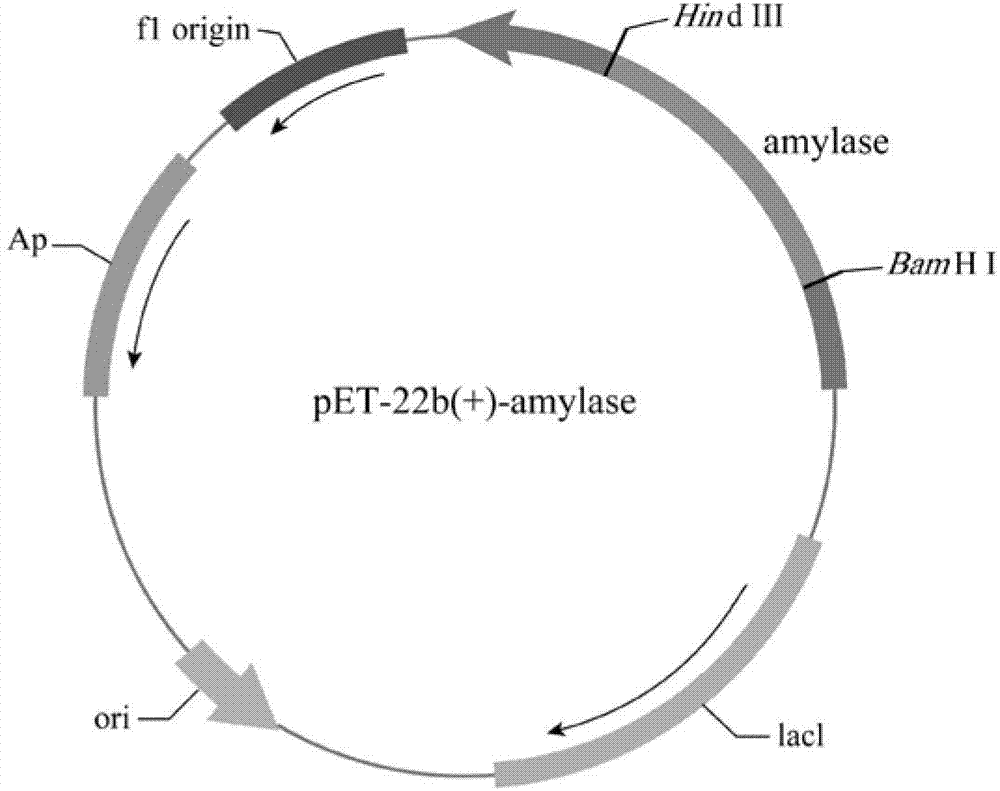
[0020] According to the amylase sequence (SEQ ID NO.1) of Bacillus alcalophilus, the amylase sequence (SEQ ID NO.1) was fully synthesized by chemical synthesis and then cloned into the plasmid pET-22b(+) to construct the recombinant plasmid pAAQ ( figure 1 ).
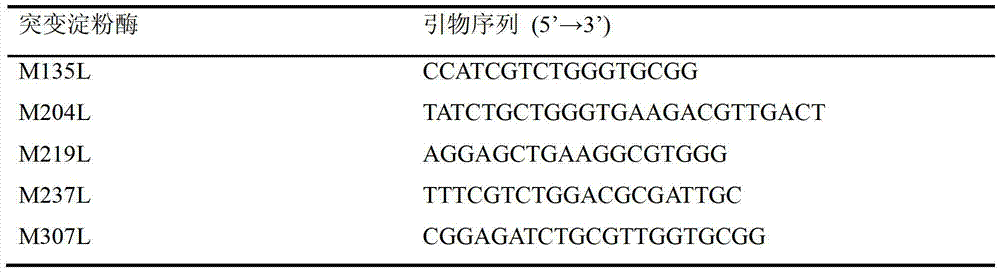
[0021] For site-directed mutagenesis of different Met sites, corresponding site-directed mutagenesis primers were designed (Table 1). Using site-directed mutagenesis primers, amyl...
Embodiment 2
[0024] Embodiment 2: the mensuration of amylase enzyme activity
[0025] Determination of Alkaline Amylase Activity by DNS Method
[0026] 1) DNS reagent configuration: Weigh 2.5g of 3,5-dinitrosalicylic acid and dissolve it in a small amount of water, add 0.5g of phenol, then dissolve 0.075g of sodium sulfite, 2.5g of sodium hydroxide, and 50g of potassium sodium tartrate, and transfer it to Put it into a 500mL volumetric flask, shake it up to volume, store it in a brown bottle and place it in a refrigerator at 4°C until use.
[0027] 2) Preparation of maltose standard curve: Prepare maltose solutions with different concentrations from 0.2g / L to 1.0g / L. Take 1mL of different concentrations of maltose and mix it with the same volume of DNS solution, put it in a boiling water bath, and keep the water bath for 10min. Cool with cold water, dilute to 10mL, A 540 Measure the absorbance. Take the concentration of maltose as the abscissa and the absorbance as the ordinate to make...
Embodiment 3
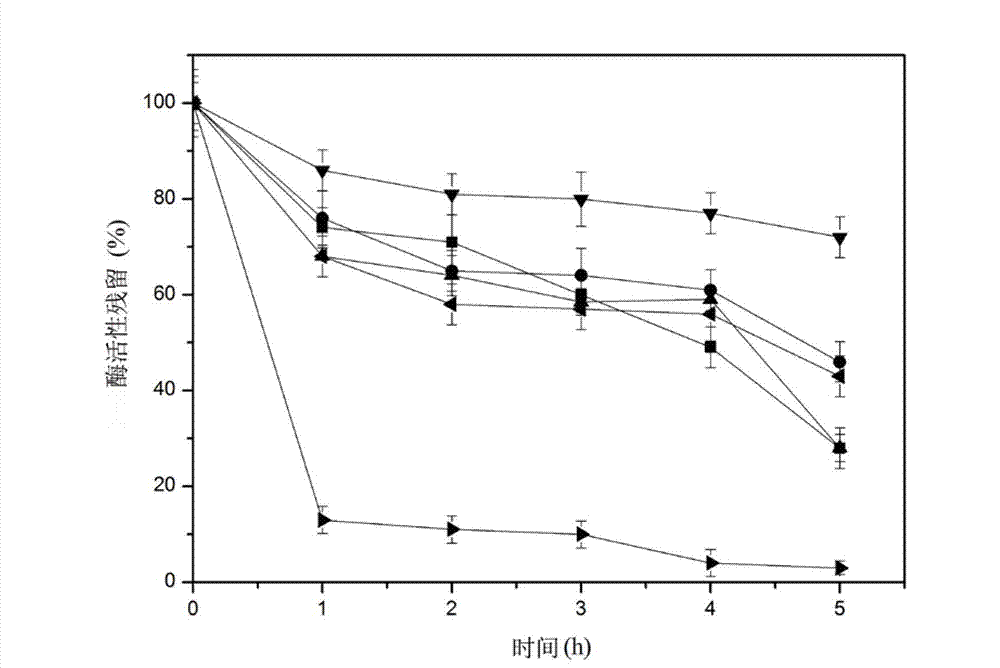
[0030] Embodiment 3: amylase is in 500mM H 2 o 2 Determination of residual enzyme activity under different conditions
[0031] In 500mM H 2 o 2 Treated at 40°C for different periods of time. Use catalase (1200U / mL) to degrade residual H 2 o 2 . Use pH10.0 glycine-sodium hydroxide buffer solution, mix the buffer solution with soluble starch, and use the method 2) to measure the remaining enzyme activity after amylase treatment (see figure 2 ). After M135, M204, M219, M237, M307 are mutated into Leu, the concentration of 500mM H 2 o 2 Treat at 40°C for 5h. After 5 hours of treatment, the remaining enzyme activity was 28%, 46%, 28%, 72%, and 43% of that before treatment. The oxidation resistance of the amylase is greatly improved, and the amylase can efficiently degrade starch in a strong oxidizing environment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com