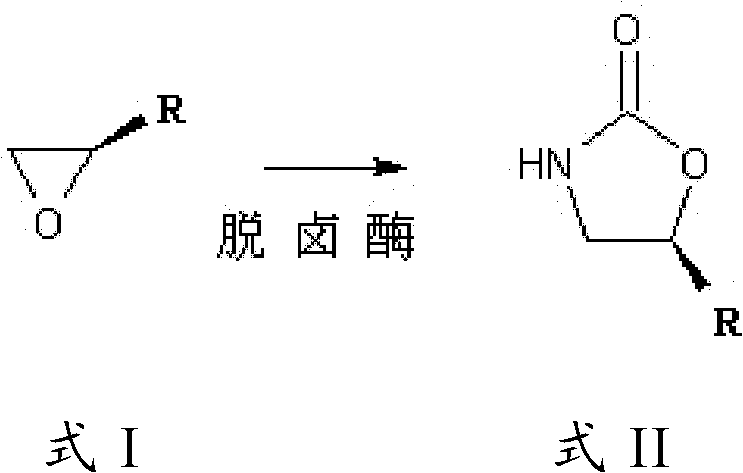
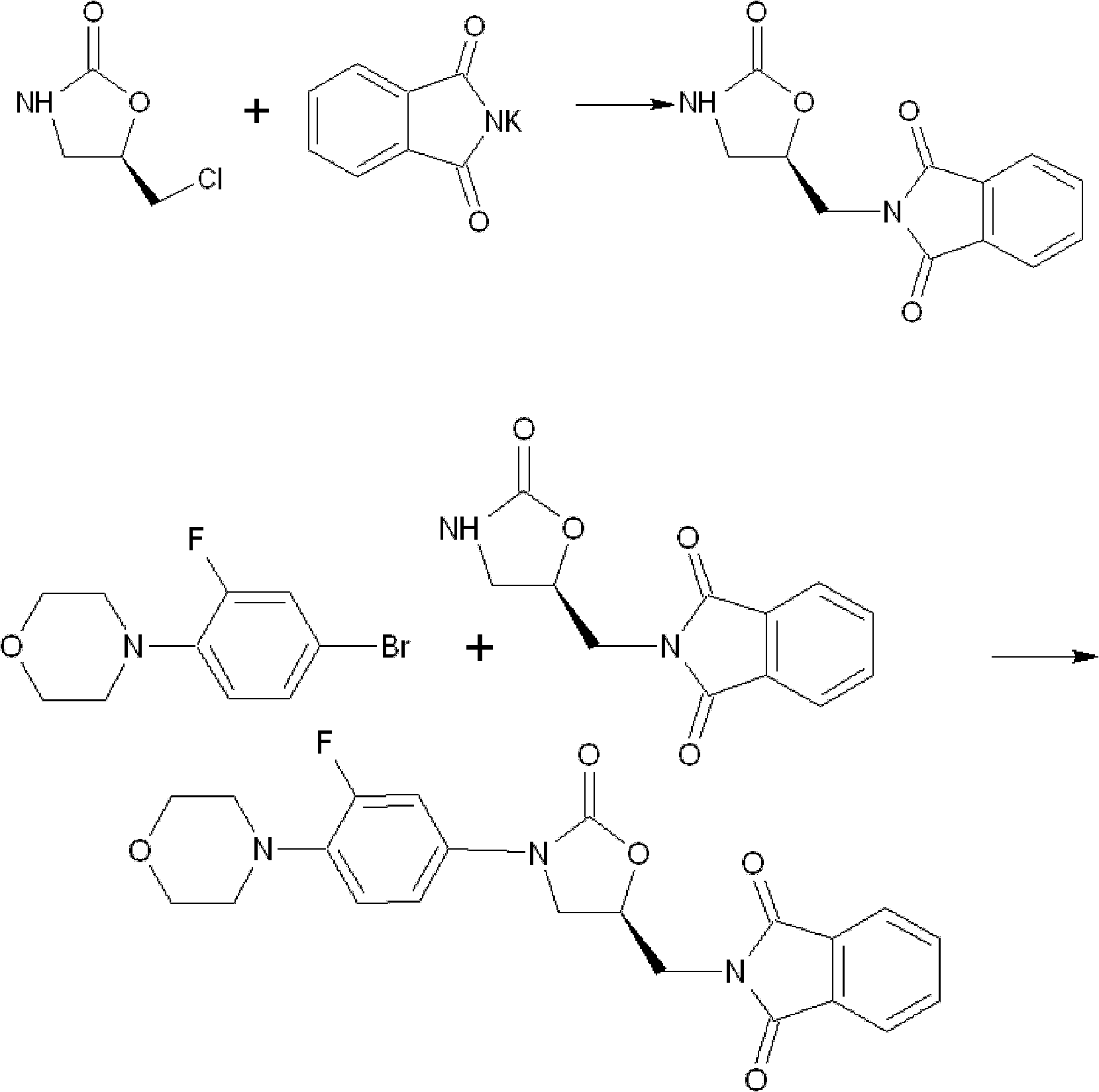
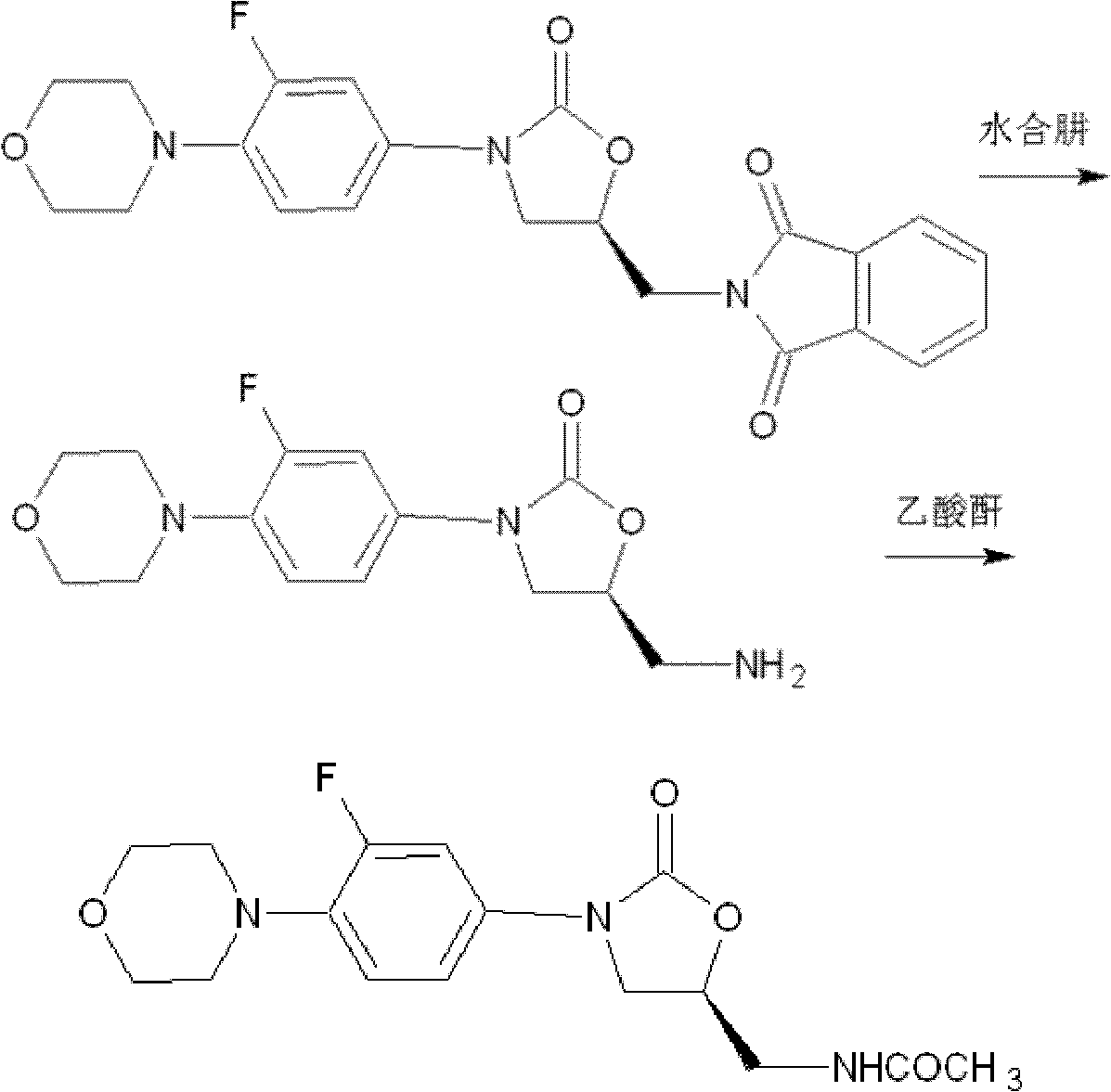
Synthesis method of linezolid intermediate
A compound and water volume technology, applied in the field of synthesis of linezolid intermediates, can solve problems such as low yield, and achieve the effects of high reaction yield, cheap and readily available raw materials, and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the fermentation preparation of dehalogenase
[0043] (1) Seed tank fermentation:
[0044] Medium components: peptone 10-15g / L, yeast extract 5-20g / L, glucose 8-20g / L (or glycerol 5-10g / L), KH 2 PO 4 10-15g / L, (NH 4 ) 2 HPO 4 4.0-5.0g / L, (NH 4 ) 2 SO 4 1-2g / L, MgSO 4 ·7H 2 O 1-1.5g / L, citric acid 1.5-2.0g / L, CoCl 2 ·6H 2 O 2-3mg / L, MnCl 2 4H 2 O 12-15.0mg / L, CuCl 2 4H 2 O 1-2mg / L, biotin 4-5mg / L, vitamin B1 4-5mg / L.
[0045] Base medium preparation: peptone, yeast extract, KH 2 PO 4 , (NH 4 ) 2 HPO 4 , (NH 4 ) 2 SO 4 , citric acid, CoCl 2 ·6H 2 O, MnCl 2 4H 2 O, CuCl 2 4H 2 O was stirred and dissolved, and the pH of the solution was adjusted to 6-7 with NaOH, kept at 121°C for 30 min, and cooled for later use. Glucose (or glycerol), MgSO 4 ·7H 2 O is sterilized separately and kept at 121°C for 20min. Biotin and vitamin B1 were sterilized by sterile membrane filtration. Then sterile glucose (or glycerol), MgSO 4 ·7H 2 O...
Embodiment 2
[0056] Embodiment 2: Preparation of 5-chloromethyl-2-oxazolidinone
[0057] synthetic route:
[0058]
[0059] Add 500ml of water into a 1000ml four-necked flask equipped with mechanical stirring, dissolve 32g of sodium cyanate, adjust the pH to 8 with 20% dilute sulfuric acid, add 25g of S-epichlorohydrin, slowly heat to 50°C, and add Halogenase 12.5 million units, control the pH at 8 during the reaction process, add 3g of activated carbon and stir for 30 minutes after the reaction for 8 hours, filter with suction, evaporate the filtrate to dryness under reduced pressure, dissolve and evaporate the dry matter with 1000ml methanol, and remove the insoluble substance by suction filtration. The filtrate was distilled under reduced pressure to obtain 40 g of the product, and the content of the target product was detected to be 84%, and the yield was 90%.
[0060] The hydrogen NMR spectrum of the reaction product ( 1 HNMR) detection and identification are correct.
Embodiment 3
[0065] Embodiment 3: the comparison of the yield of 5-chloromethyl-2-oxazolidinone prepared under different conditions
[0066] Synthetic route: with embodiment 2
[0067] According to the synthesis method of Example 2, different experimental conditions were changed, such as reaction temperature, reaction time, reaction pH value, concentration of added substrate and enzyme consumption, to compare the yield of the product obtained under each reaction condition. The results are shown in Table 1.
[0068] Comparison of product yields under different conditions in table 1
[0069]
[0070] The experimental results show that the concentration and temperature of the reaction substrate have a great influence on the reaction. From reactions 4 and 5, it can be seen that under the same conditions, the yield of low-concentration substrate is higher, this is because the substrate has a great influence on the activity of the enzyme, and the reason that the concentration is too high wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com