Alprostadil injection
A technology of dil injection and alprostadil, applied in the direction of medical preparations with non-active ingredients, blood diseases, extracellular fluid diseases, etc., can solve the problems such as the complicated preparation process of fat emulsion and the influence of drug stability, so as to avoid milk Effects of droplet aggregation, prevention of oxidation reaction, and improvement of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The alprostadil non-aqueous injection provided by the present embodiment includes:
[0037] Alprostadil 0.001-0.05mg / mL, oil phase 0.01-100mg / mL, phospholipid 10-500mg / mL, and the rest are organic solvents.
[0038] In the above prescription, the phospholipids include at least one of the following: natural phospholipids and synthetic phospholipids.
[0039] The oil phase includes at least one of the following: soybean oil, corn oil, castor oil, olive oil, coconut oil, peanut oil, fish oil, cottonseed oil, glyceryl monostearate, glyceryl monooleate, sesame oil, red Flower Oil, Medium Chain Triglycerides, Ethyl Oleate.
[0040] The organic solvent includes at least one of the following: ethanol, glycerin, propylene glycol, polyethylene glycol.
[0041] The medium chain triglycerides (Medium chain triglycerides, MCT) mentioned in this example refers to the esterification of fatty acids containing 6-12 carbon atoms by glycerol. Wherein preferred MCT refers to saturated c...
Embodiment 2 Embodiment 10
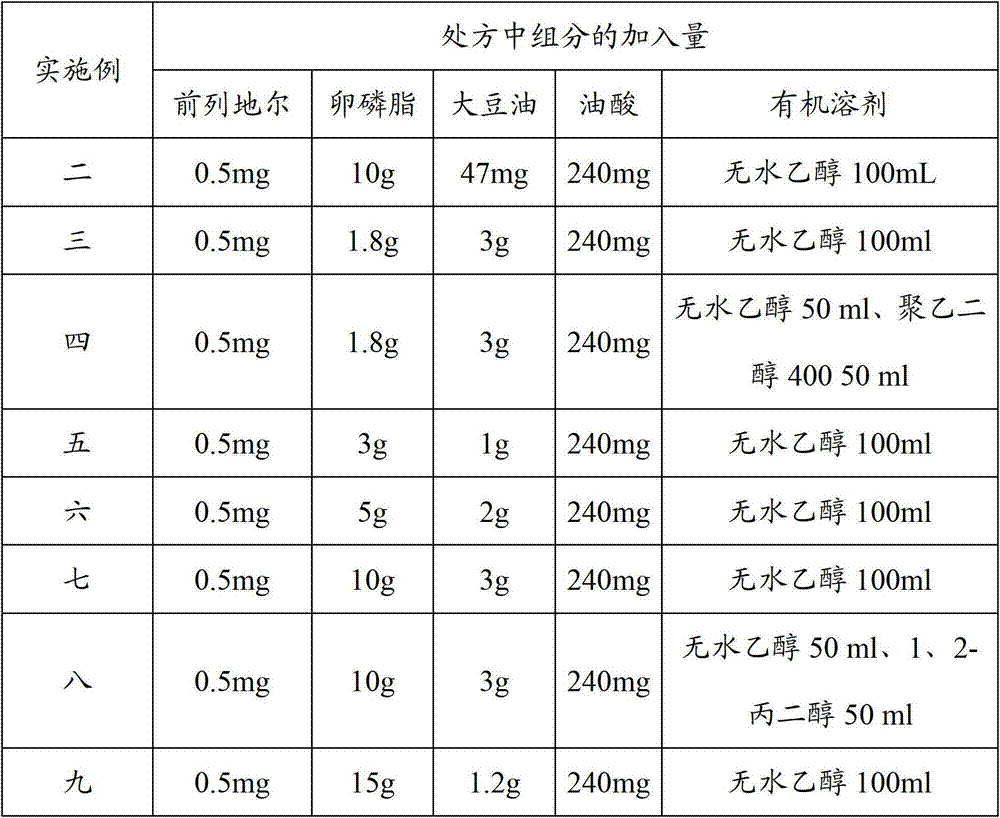
[0063] Following table 1 is the prescription of the alprostadil injection that embodiment two to ten provides.
[0064] The preparation methods of the above-mentioned nine embodiments are basically similar, for example, adding alprostadil to an appropriate amount of absolute ethanol or other organic solvents, and after completely dissolving, add lecithin, soybean oil, oleic acid and the remaining prescription amount of absolute ethanol , stir at room temperature to form a transparent and clear liquid composition after mixing them uniformly, and the order of adding or dissolving each component can be changed according to the actual prescription.
[0065] The alprostadil injection prescription of table 1 embodiment two to ten
[0066]
[0067]
[0068] In the above-mentioned embodiment 2, in clinical application, the injection is diluted 1 to 100 times before use, and the effect is better, and it is more preferable to dilute it 10 times.
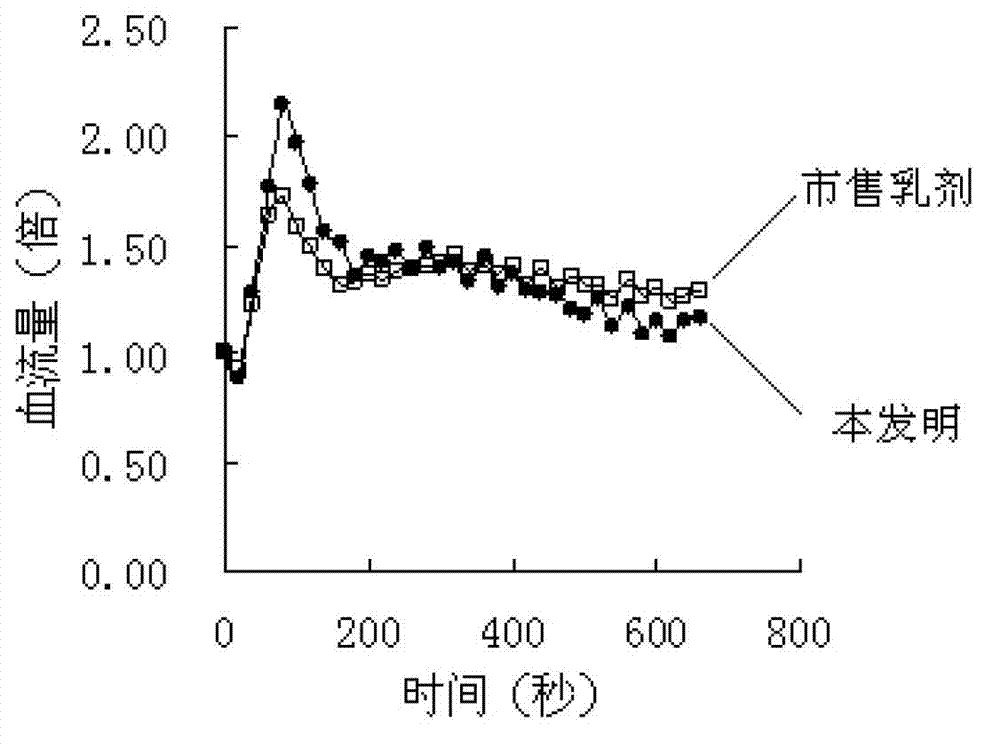
[0069] In order to further illus...
Embodiment 11
[0071] Determination of Particle Size of Alprostadil Injection
[0072] experiment method:
[0073] Take 0.1 mL of the injection prepared in Example 2, add purified water (pre-filtered with a membrane with a pore size of 0.22 μm) and dilute it 5000 times, mix well, and use it as the test solution, use a Malvern laser particle size analyzer (Zetasizer 3000HS, Malvem, UK ) to measure the particle size and distribution of the above liquid to be tested at 25°C.
[0074] result:
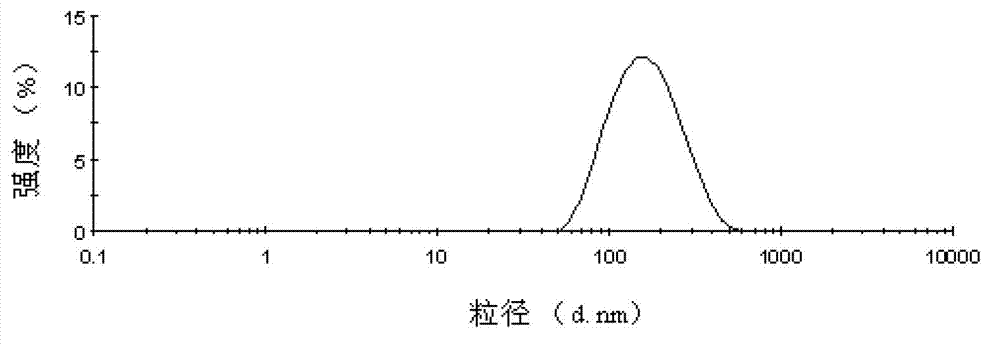
[0075] Particle size distribution see figure 1 , the average particle size is 146.0nm, and the PDI is 0.177. It can be seen that the injection provided by the invention is suitable for intravenous injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com