Mouth mucosa sustained-release preparation for treating oral and periodontal diseases by chlorhexidine
A technology for oral periodontal disease and oral mucosa, which is applied in the field of medicines for treating oral mucosal diseases and the preparation thereof, can solve the problems of short drug release time, loss of treatment effect of oral patients, etc., and achieves long drug release time and convenient application. , the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Pharmaceutical active adhesive layer (a) raw material:
[0042] Adhesive: 8.0 grams of polyvinylpyrrolidone, 8.0 grams of polyoxyethylene;
[0043] Disintegrant: microcrystalline cellulose 10.0 g;
[0044] Flavoring agent: menthol 1.5g;
[0045] Antibiotics: minocycline 2.5 grams;
[0046] Raw material of non-adhesive layer (b): composed of polyvinyl alcohol (alcoholysis degree 92%).
[0047] Preparation of Oral Mucosal Drug Sustained Release Patch:
[0048] A flat-bottomed mold with a diameter of 5 mm is used to prepare the tablet by direct compression, and the dried powder of the above-mentioned excipients and the dry powder of the active drug are uniformly mixed according to the formula ratio, and a flat cylinder is obtained under the direct compression condition of 1000 kg of pressure and 6 seconds. Shaped patch, the thickness of the patch is 0.8-1.2 mm, and the weight of the patch is 25.0-32.0 mg;
[0049] Prepare polyvinyl alcohol into a 10% aqueous solution ...
Embodiment 1-1
[0052] Example 1-1: Single-sided sealing film patch experiment
[0053] The sample diameter is 5.0 mm, the thickness is 1.2 mm, and the weight is 30 mg
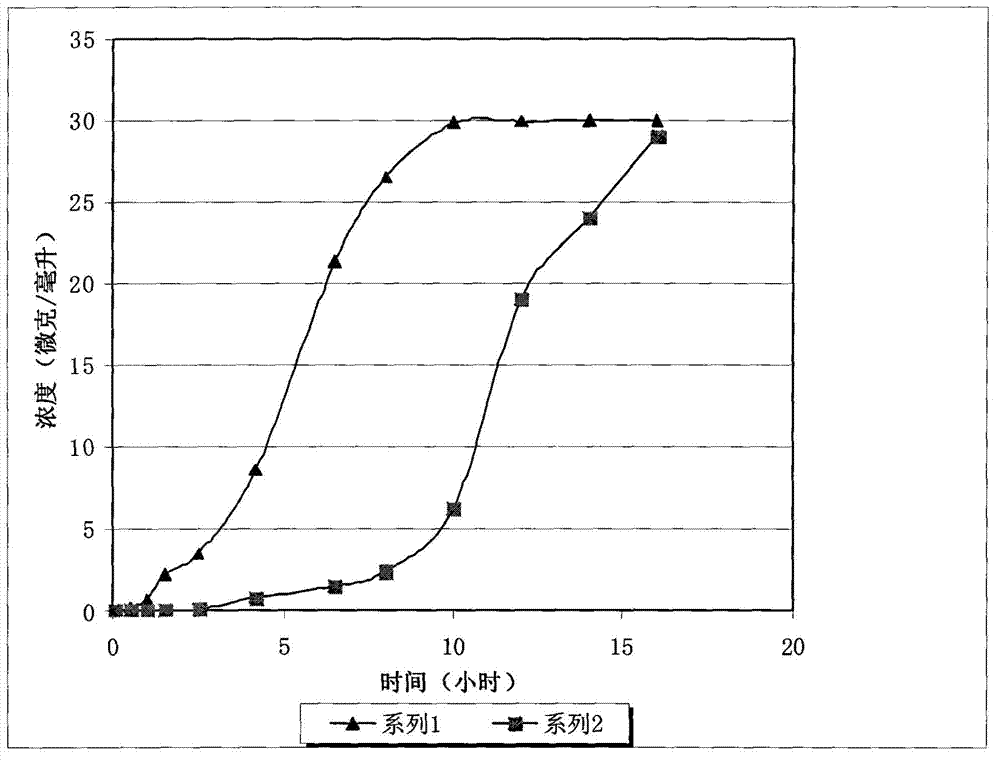
[0054] figure 1 Series 1 shows the results of the dissolution test for this patch. It can be seen that the solid concentration in the dissolution medium increases steadily over time, and its sustained release time can reach 8 hours. The drug release amount in the early stage is equal, and the drug release amount between 3 hours and 7 hours is enlarged. In 7-8 hours, the drug ingredients in the patch are almost released, and the drug release rate is equivalent to that of the previous period, and the disintegration process of the entire drug patch is relatively stable.
Embodiment 1-2
[0055] Example 1-2: Overall coating comparison sheet experiment
[0056] The sample piece has a diameter of 5.0 mm, a thickness of 1.2 mm, and a weight of 30 mg.
[0057] figure 1Series 2 shows the results of the dissolution test for this patch. It can be seen that there is basically no substance dissolved in the patch in the first 5-6 hours, the dissolved amount increases significantly in 7 hours, and basically completely dissolves in 15 hours.
[0058] The above experimental results show that the polyethylene coating coated on the patch has an obvious restrictive effect on the dissolution of the adhesive.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com