Conjugated polymer and application thereof in hybridization of solar battery
A conjugated polymer and heteroarylene technology, applied in the field of organic polymer semiconductor materials, can solve the problem of high energy conversion efficiency of only 3.78%, and achieve high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
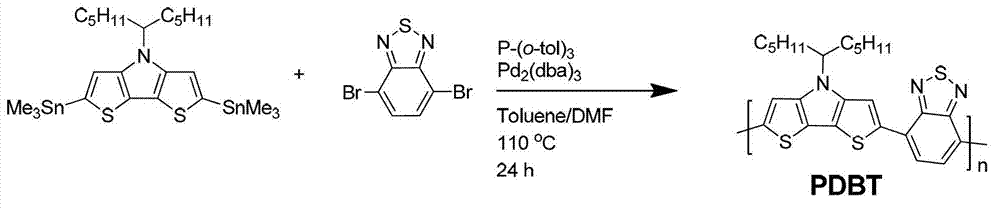
[0040] Polymer: Preparation of poly 4-(undecyl-6-)-4-hydrogen-dithienopyrrole-co-2,1,3-benzothiadiazole (remember polymer PDBT), synthetic route like figure 1 shown. The polymer structure obtained is:
[0041]
[0042] Take 0.25 grams of 2,6-bis(trimethylstannyl)-4-(undecyl-6-)-4-hydrogen-dithienopyrrole, 0.11 grams of 4,7-dibromo-2,1, Add 3-benzothiadiazole to a 50 ml reaction tube, add catalyst 0.01 g tris(dibenzylideneacetone) dipalladium, ligand 0.02 g tri-o-methylphenylphosphine, add 5 ml anhydrous toluene, 0.5 ml Anhydrous N,N-dimethylformamide was stirred for 24 hours in an argon atmosphere at 110°C. Cool the polymer to room temperature and slowly pour it into 70 ml of methanol. The precipitated polymer is filtered and washed with methanol and n-hexane successively in a Soxhlet extractor. Finally, it is dissolved in chloroform and precipitated into methanol, filtered. , dried under vacuum at 100°C for 12 hours to obtain a deep purple solid powder polymer with th...
Embodiment 2
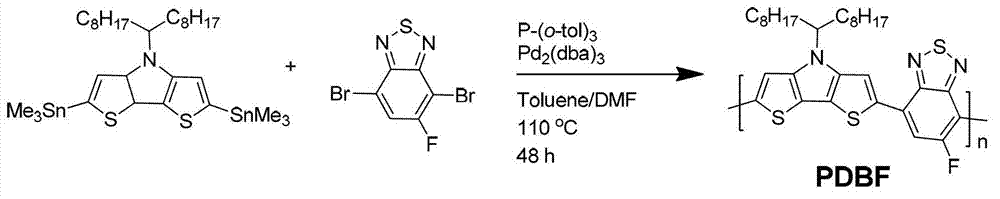
[0044] The preparation of polymer poly 4-(heptadecyl-9-)-4-hydrogen-dithienopyrrole-co-2,1,3-fluorobenzothiadiazole (polymer PDBF), the synthetic route is as follows figure 2 shown. The polymer structure obtained is:
[0045] .
[0046] Take 0.25 grams of 2,6-bis(trimethyltin base)-4-(heptadecyl-9-)-4-hydrogen-dithienopyrrole, 0.10 grams of 1,3-dibromo-5-(4 -octylphenyl)-5H-thiophene-[3,4-c]-pyrrole-3,6-dione was added to a 50 ml reaction tube, and 0.01 g of catalyst tris(dibenzylideneacetone) dipalladium was added to prepare 0.02 g of tri-o-methylphenylphosphine, 5 ml of anhydrous toluene and 0.5 ml of anhydrous N,N-dimethylformamide were added, and the reaction was stirred for 48 hours under an argon atmosphere at 110°C. Cool the polymer to room temperature and slowly pour it into 70 ml of methanol. The precipitated polymer is filtered and washed with methanol and n-hexane successively in a Soxhlet extractor. Finally, it is dissolved in chloroform and precipitated into...
Embodiment 3
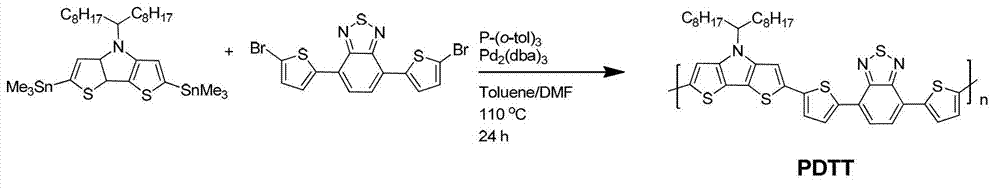
[0048] Polymer poly 4-(heptadecyl-9-)-4-hydrogen-dithienopyrrole-co-5,5-(4,7-di-2-thienyl-2,1,3-benzo Thiadiazole) (Polymer PDTT), the synthetic route is as follows image 3 shown. The polymer structure obtained is:
[0049] .
[0050] Take 0.25 g of 2,6-bis(trimethyltinyl)-4-(heptadecyl-9-)-4-hydrogen-dithienopyrrole, 0.15 g of 4,7-dibromo-2-thienyl -2,1,3-Benzothiadiazole was added to a 50 ml reaction tube, 0.01 g of tris(dibenzylideneacetone) dipalladium was added as a catalyst, 0.02 g of tri-o-methylphenylphosphine was added as a ligand, and 5 ml of Water toluene, 0.5 ml of anhydrous N,N-dimethylformamide, stirred and reacted in an argon atmosphere at 110°C for 24 hours. Cool the polymer to room temperature and slowly pour it into 70 ml of methanol. The precipitated polymer is filtered and washed with methanol and n-hexane successively in a Soxhlet extractor. Finally, it is dissolved in chloroform and precipitated into methanol, filtered. , vacuum drying at 100°C fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dispersion | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
| Dispersion | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com