Novel glycerol dehydration catalyst and production method therefor
A manufacturing method and catalyst technology, applied in the direction of catalyst activation/preparation, organic chemical methods, chemical instruments and methods, etc., can solve the problems of unusable acrolein yield and achieve high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1 (preparation of NbO x )
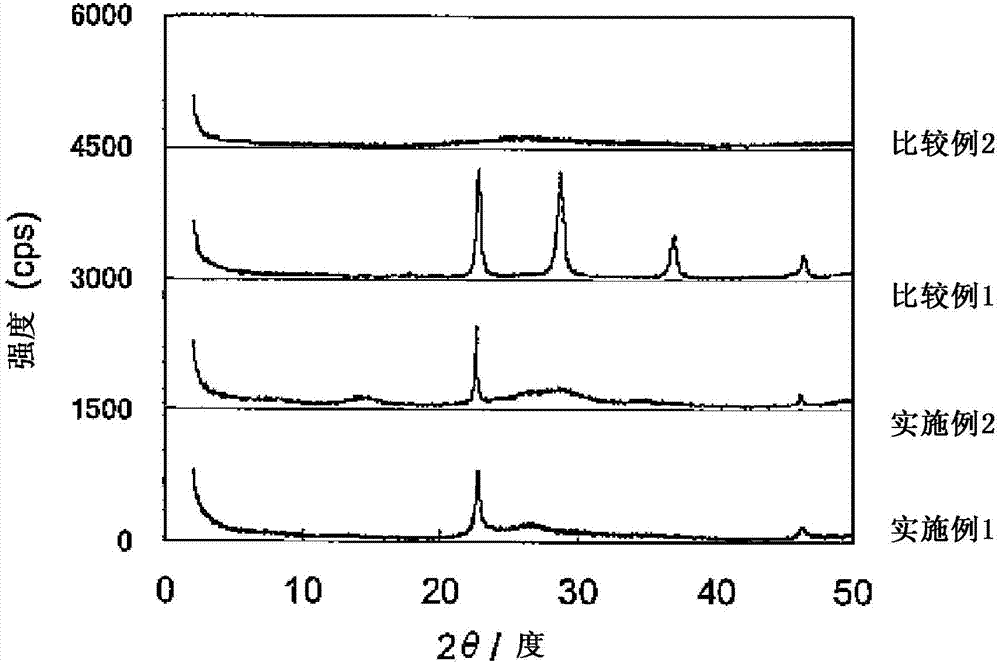
[0067] 7.4529g niobium hydrogen oxalate (Nb(HC 2 o 4 ) 5 ·nH 2 O) Disperse in 25ml of distilled water and ultrasonically vibrate for 10 minutes. The resulting dispersion was introduced into an autoclave and subjected to hydrothermal synthesis at 175 °C for three days. The resulting slurry was vacuum filtered, washed with distilled water and then dried overnight at 80 °C to obtain NbO x catalyst powder. figure 1 The X-ray diffraction pattern of the obtained catalyst is shown. figure 1 A peak is shown at 2θ=22.6 and 2θ=46.1 each.
[0068] Catalyst Reactivity Evaluation
[0069] The reactivity of the resulting catalysts was evaluated in a fixed bed operated by atmospheric pressure of the reactants. The catalyst powder is press-molded and passed through a sieve to obtain particles with a particle diameter of 9 to 12 mesh. 10 cc of particles were packed in a reaction tube (20 mm in diameter) made of SUS to form a catalyst be...
Embodiment 2
[0074] Example 2 (Preparation W 0.5 Nb 1.0 o x )
[0075] 1.1376g niobium oxide (Nb 2 o 5 ·nH 2 O) Disperse in 25 ml of distilled water and ultrasonically vibrate for 10 minutes to prepare a niobium oxide dispersion. In a separate flask, 0.7660 g of ammonium tungstate ((NH 4 ) 6 [H 2 W 12 o 40 ] nH 2 O) Dissolve in 20 ml distilled water. The obtained ammonium tungstate aqueous solution was slowly added to the niobium oxide dispersion. The resulting mixture was introduced into an autoclave and subjected to hydrothermal synthesis at 175 °C for three days. The resulting slurry was vacuum filtered, washed with distilled water and then dried overnight at 80 °C to obtain NbO x catalyst powder. The catalyst shows figure 1 The X-ray diffraction pattern, which has a peak at 2θ=22.6 and 2θ=46.1. This confirms that the obtained crystal structure is consistent with the NbO x similar.
[0076] Catalyst Reactivity Evaluation
[0077] The reactivity of the obtained cata...
Embodiment 3
[0078] Example 3 (Making Cs exchanged for W 0.5 Nb 1.0 o x )
[0079] Ion exchange of Cs was performed as follows: 0.2612 g of CsCl was added to 25 ml of distilled water. With the WNbO obtained in 1.0g embodiment 2 x It was added to the resulting aqueous CsCl solution and stirred at room temperature for 24 hours. Then, the resulting slurry was vacuum filtered, washed with distilled water and then dried overnight at 80 °C to obtain Cs-exchanged WNbO x .
[0080] Catalyst Reactivity Evaluation
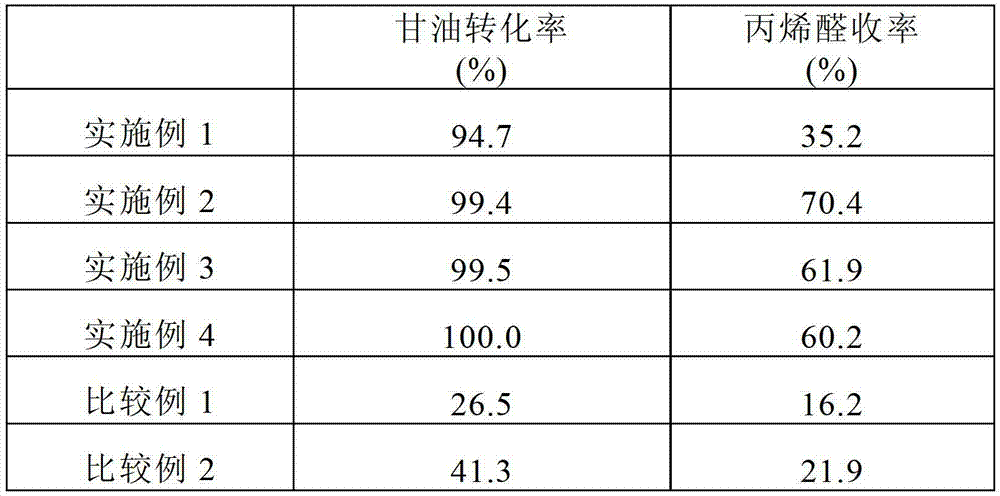
[0081] The reactivity of the obtained catalyst was evaluated by the same method as in Example 1, and the results are summarized in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com