Catalytic oxidation method of pyridine
A technology for oxidizing pyridine and pyridine, applied in directions such as organic chemistry, can solve problems such as complex production process, harmful emissions, etc., and achieve the effects of overcoming complex production process, simple production process and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0014] In the preparation method provided by the present invention, the solvent is selected from alcohols such as water or methanol, ethanol, n-propanol, isopropanol, tert-butanol, isobutanol or ketones such as acetone and methyl ethyl ketone or acetonitrile, Nitriles such as propionitrile and phenylacetonitrile or their mixtures, preferably acetonitrile, acetone, methanol, water or their mixtures, more preferably acetone, methanol and / or acetonitrile.
[0015] In the preparation method provided by the present invention, there is no special requirement on the order of addition. Pyridine may be added first, or oxidizing agent or solvent may be added first.
Embodiment 1
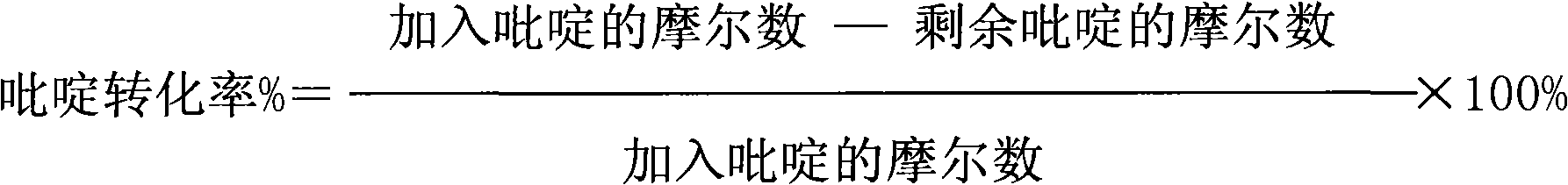
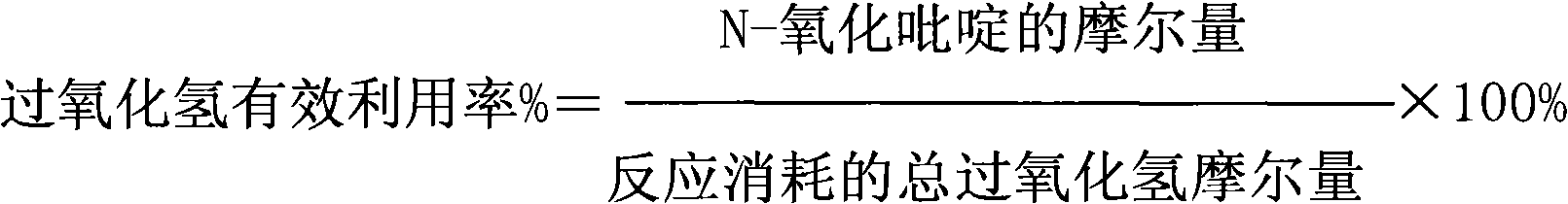
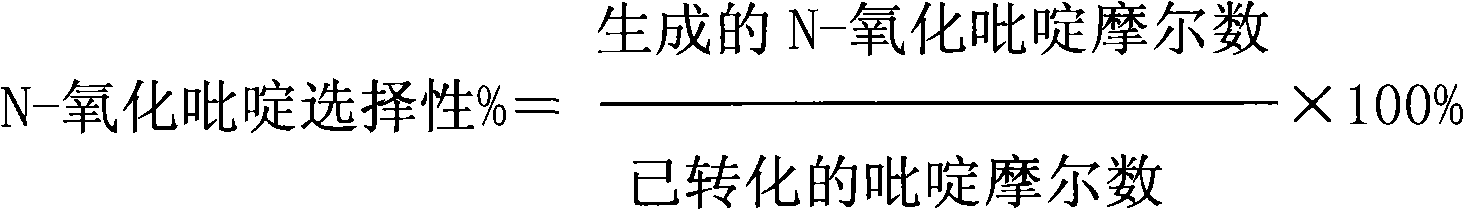
[0033] Pyridine, hydrogen peroxide, solvent and catalyst (the mol ratio of zinc nitrate and titanium silicon molecular sieve 0.2: 1) is 1: 2 according to the mol ratio of pyridine and hydrogen peroxide, solvent acetonitrile and catalyst mass ratio are 20: 1, pyridine and The catalyst mass ratio is 10:1, and the reaction is carried out at a temperature of 50° C. and a pressure of 1.5 MPa. After 2 hours of reaction, the conversion rate of pyridine was 23%, the effective utilization rate of hydrogen peroxide was 52%, and the selectivity of pyridine N-oxide was 63%.
Embodiment 2
[0035]Pyridine, hydrogen peroxide, solvent and catalyst (the mol ratio of zinc phosphate and titanium silicon molecular sieve 1: 1) are 1: 3 according to the mol ratio of pyridine and hydrogen peroxide, solvent acetone and catalyst mass ratio are 50: 1, pyridine and The catalyst mass ratio is 20:1, and the reaction is carried out at a temperature of 60° C. and a pressure of 2.5 MPa. After 2 hours of reaction, the conversion rate of pyridine was 35%; the effective utilization rate of hydrogen peroxide was 57%; the selectivity of pyridine N-oxide was 62%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com