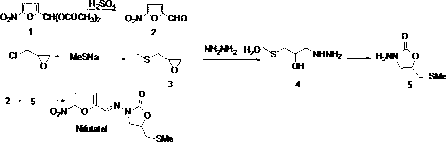
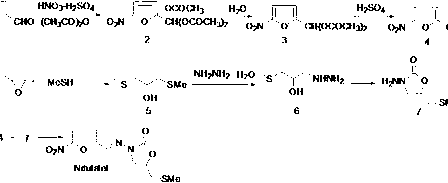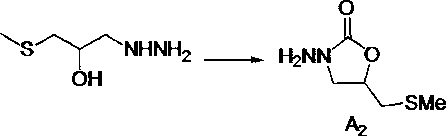Method for preparing nifuratel
A technology of nifuratel and a synthesis method, which is applied in the field of drug synthesis, can solve the problems of decreased yield, low yield of nifuratel, difficult control of high vacuum, etc., and achieves low environmental pollution and simple and easy-to-control synthesis method. , the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Pump 18.2 Kg of 80% hydrazine hydrate into a 50L clean reactor, raise the temperature of the reaction solution to 80-85°C, and gradually add 11 kg of methylthiopropylene oxide under mechanical stirring. During the dropwise addition, the reaction is violently exothermic. The reaction temperature should be strictly controlled not to exceed 95°C. After the addition, the reaction was continued for 4 hours at 90-95°C. Control the reaction liquid at 80-85°C to distill hydrazine hydrate under reduced pressure (vacuum degree control ≤0.09MPa), carry out reduced-pressure distillation under this vacuum condition until no liquid is evaporated; then cool the reaction kettle to 60-65°C to remove Pressure device, add 6 kg of absolute ethanol, stir for 20 minutes, then connect the decompression device for distillation, and raise the temperature to 80-85°C (vacuum degree control ≤0.09MPa) until no liquid evaporates; repeat the above operation of 6 kg of absolute ethanol Twice, a total...
Embodiment 2
[0039] Add 7.3 kg of anhydrous methanol into a 25L plastic bucket, add 1.3 kg of sodium methoxide under stirring, and dissolve to obtain a methanol solution of sodium methoxide for use (the reaction liquid has no temperature limit, but anhydrous operation must be ensured); in intermediate A 1 12.4 kg of diethyl carbonate was added to the 50L reaction kettle under stirring, and after stirring for 15 minutes, the prepared sodium methoxide methanol solution was added dropwise to the reaction solution, and the dropwise addition was completed in 0.5 hours, and then the temperature was raised to 60-70°C The reaction was refluxed for 3 hours. Cool the reactor to 20-25°C, then add 8.0 kg of absolute ethanol and stir for 15 minutes to obtain A 2 ethanol solution, set aside.
Embodiment 3
[0041]Pump 47.0 kg of water into a 100L reactor, gradually add 5.5 kg of concentrated sulfuric acid under stirring, and stir the reaction solution evenly to obtain a 10% dilute sulfuric acid solution for subsequent use; pump 44.0 kg of 95% ethanol into a 200L reactor, stir 5-nitrofurfural diethyl ester (B 1 ) 25.0 kg was added to the reaction kettle, and the reaction solution was fully stirred evenly, and then the prepared 10% dilute sulfuric acid solution was gradually added dropwise. The reaction kettle was lowered to room temperature for use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com