Vanadium oxide complex as well as preparation method and application thereof
A technology of complexes and vanadyl, which is applied in the direction of pharmaceutical formulations, chemical instruments and methods, and medical preparations containing active ingredients, etc., can solve problems that have not been reported, and achieve easy-to-obtain raw materials, activity inhibition, and simple synthesis methods Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
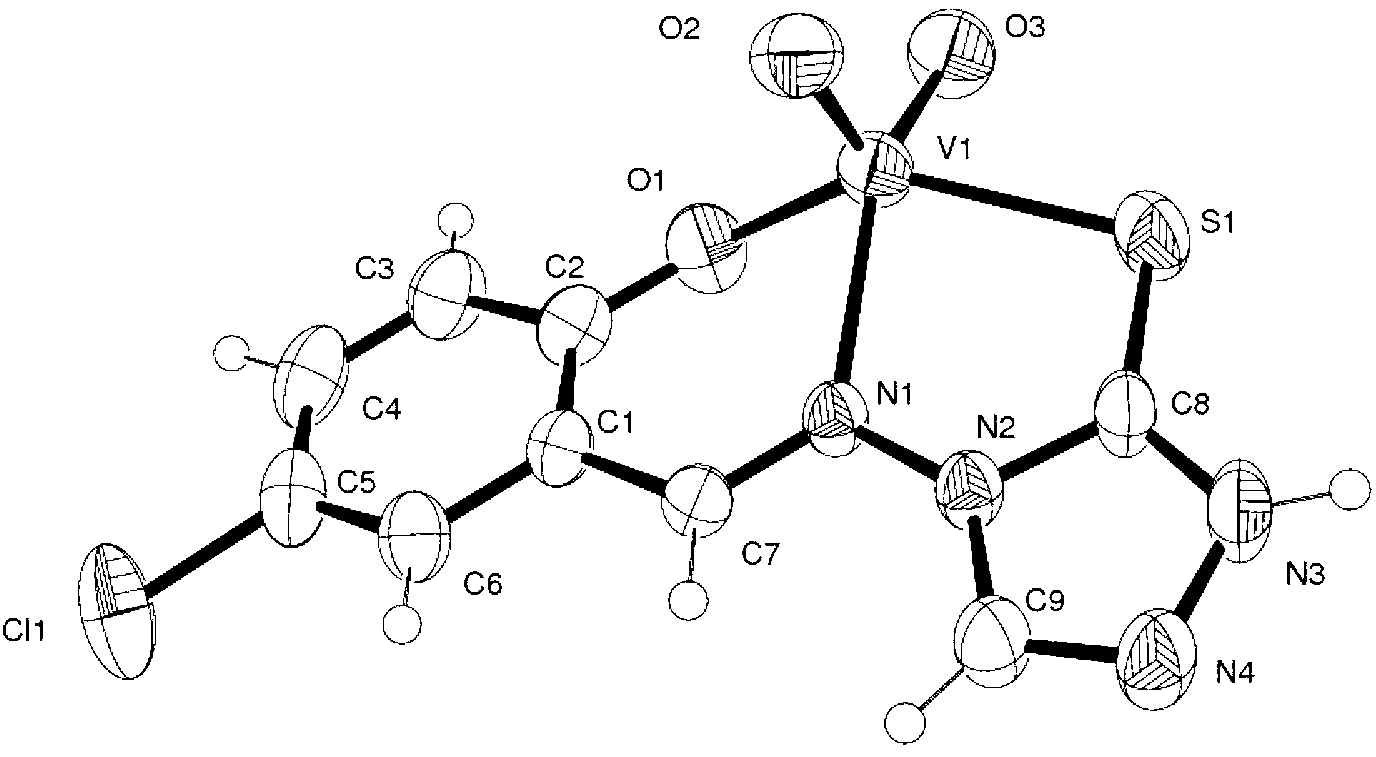
[0025] Preparation of complexes
[0026] (1) Synthesis of 4-amino-5-mercapto-1,2,4-triazole: Take 10mmol (1.06g) of bisthiosemicarbazide in a round bottom flask, add 10mL of formic acid solution, stir and reflux for 4h, cool to room temperature, Unreacted formic acid was distilled off under reduced pressure, washed with water to obtain a crude product, and recrystallized with water to obtain a white crystalline product;
[0027] (2) Synthesis of 5-chlorosalicylaldehyde-4-amino-5-mercapto-1,2,4-triazole Schiff base ligand: Take 5mmol (0.58g) of 4-amino-5-mercapto-1, Add 50ml of absolute ethanol to dissolve 2,4-triazole in a round bottom flask, add dropwise 5mmol (0.78g) of 5-chlorosalicylaldehyde in absolute ethanol solution 40ml and 0.4ml of concentrated sulfuric acid under stirring, a large amount of light Yellow precipitate, after heating to reflux for 4 hours, cooling, filtering, recrystallization of solid powder in absolute ethanol, and precipitation of light yellow micro...
Embodiment 2
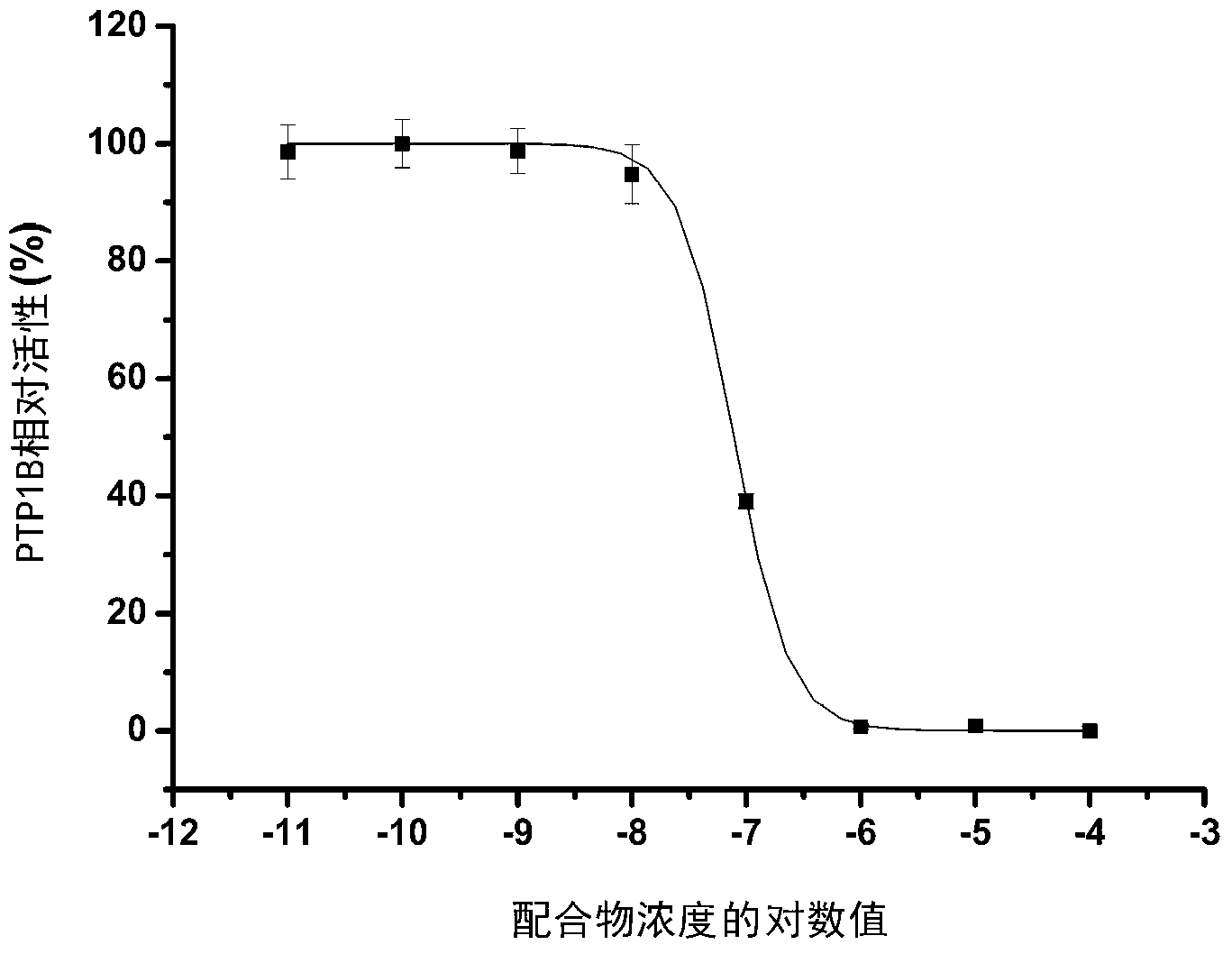
[0038] Example 2 Detection of the inhibitory effect of the vanadyl complexes of the present invention on PTP1B.
[0039]The vanadyl complex crystals were dissolved in dimethyl sulfoxide (DMSO) to prepare 10 -2 The mother liquor of M was diluted with DMSO into solutions of different concentrations (10 -4 M, 10 -5 M, 10 -6 M, 10 -7 M, 10 -8 M, 10 -9 M, 10 -10 M). The enzyme activity inhibition test was carried out with 2mM p-nitrophenyl phosphate disodium salt (pNPP) as the reaction substrate in the MOPS buffer system [20mM morpholinopropanesulfonic acid (MOPS), 50mM NaCl] at pH 7.20 , the experimental method is as follows: this experiment is carried out in a 96-well plate, 83 μl of enzyme-containing buffer solution, 10 μl of inhibitors with different concentration gradients are sequentially added, and 2 μl (0.1M) of pNPP is used to start the reaction, and after standing for about 15 minutes, add 5 μl (2M) of NaOH was used to stop the reaction, and the degradation produc...
Embodiment 3
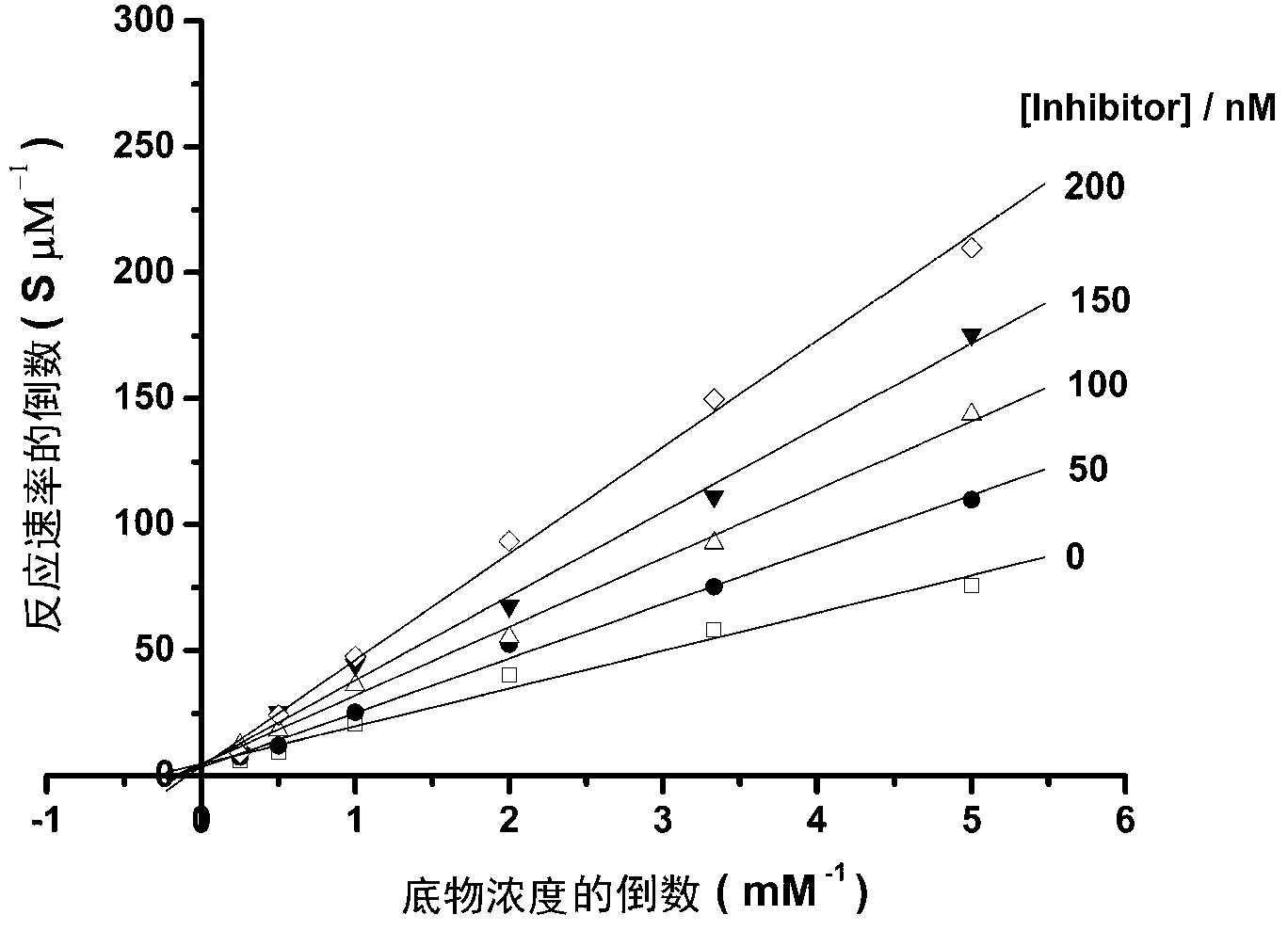
[0040] Example 3 Determination of the type of inhibition of PTP1B by the vanadyl complexes of the present invention.
[0041] The vanadyl complex crystals were dissolved in dimethyl sulfoxide (DMSO) to prepare 10 -2 The mother solution of M was diluted with DMSO into solutions of different concentrations (200nM, 100nM, 50nM, 25nM), and the concentrations of the substrate pNPP were 4mM, 2mM, 1mM, 0.5mM, 0.3mM, 0.2mM. The experimental reaction system is: 88 μl enzyme-containing (PTP1B) buffer solution containing: 20 mM morpholinopropanesulfonic acid (MOPS), 50 mM NaCl, 10 μl inhibitors with different concentration gradients, and 2 μl different concentrations of pNPP are added to the reaction system to start the reaction. Measure the change in light absorption. Measure the absorbance value of each inhibitor concentration to each substrate concentration, according to the formula A=εbc (b=1cm, ε=1.78×10 4 , A is the absorbance at 405nm, and C is the reactant concentration mol / L) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com