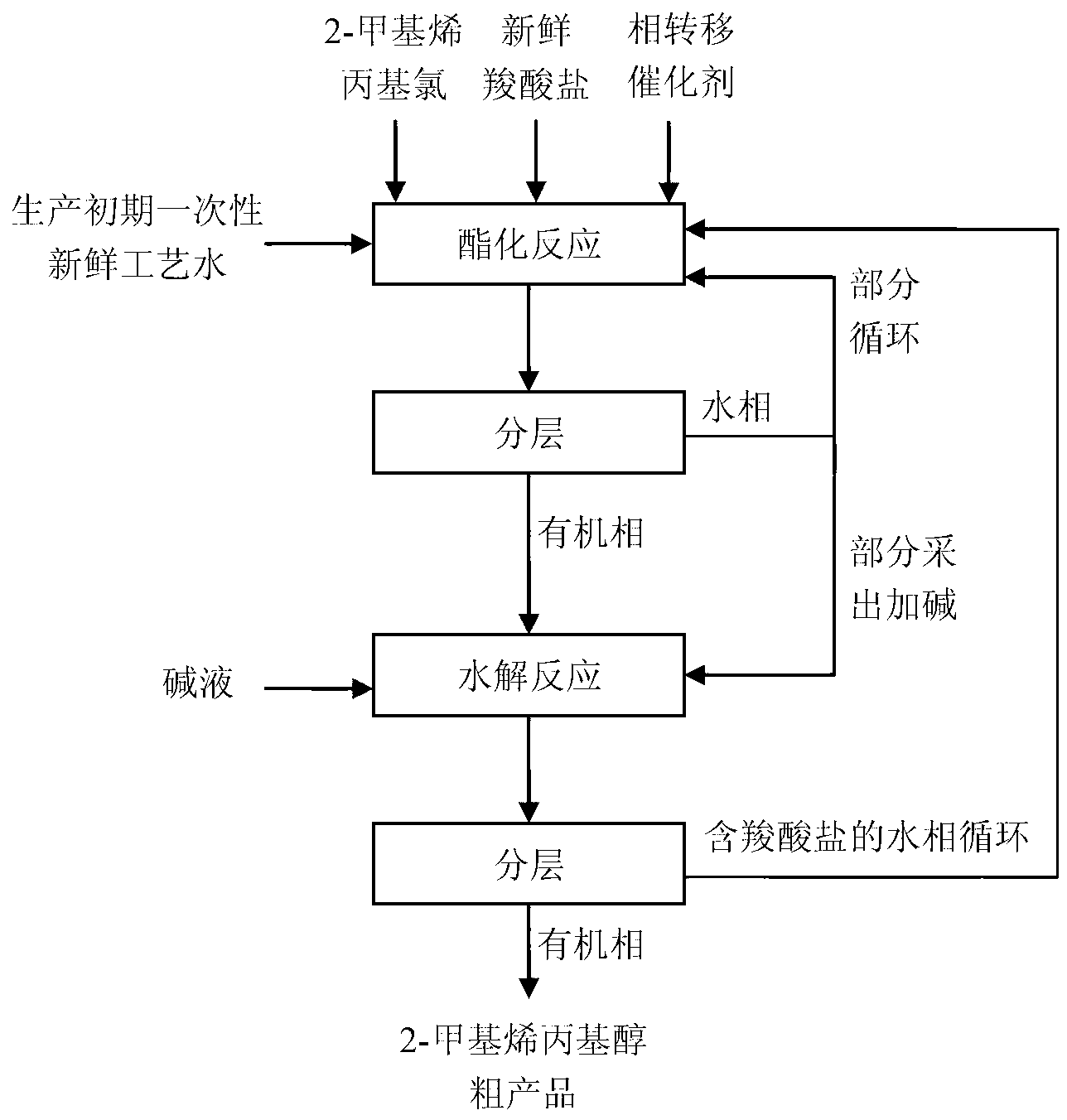
Method for preparing 2-methyl allyl alcohol by esterification and hydrolysis
A technology of methallyl alcohol and methallyl chloride, applied in chemical instruments and methods, preparation of oxygenated compounds, preparation of organic compounds, etc., can solve the problem of low concentration of lye, many side reactions of etherification, etc. The problem of high reaction temperature can reduce the energy consumption and material consumption of separation, improve the product yield, and achieve the effect of high product selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]In a Hastelloy autoclave, 57g of 2-methallyl chloride, 170g of water, 214g of sodium formate and 11.4g of benzyltriethylammonium chloride were added, the reaction temperature was controlled at 60°C, and the esterification reaction was carried out for 15 hours. The reaction solution was left to stand and separated to obtain 58.5 g of an organic phase and 393.9 g of an aqueous phase. Through gas chromatography analysis, in the organic phase, the mass fraction of 2-methallyl chloride is 1.2%, the mass fraction of 2-methallyl formate is 81.4%, and the mass fraction of 2-methallyl alcohol is 17.2%. Add the organic phase to a three-necked flask, add 299.6g of 40% sodium bicarbonate solution, and hydrolyze at 80°C for 0.5h. After the hydrolysis was completed, the reaction solution was allowed to stand and separate into layers to obtain 44.8 g of an organic phase and 313.3 g of an aqueous phase. Through gas chromatography analysis, the mass fraction of 2-methallyl chloride in ...
Embodiment 2
[0037] In a Hastelloy autoclave, 57g of 2-methallyl chloride, 113.3g of water, 96.9g of potassium valerate and 11.4g of polyethylene glycol were added, the reaction temperature was controlled at 120°C, and the esterification reaction was carried out for 8 hours. The reaction solution was left to stand and separated to obtain 87.7 g of an organic phase and 190.9 g of an aqueous phase. Through gas chromatography analysis, in the organic phase, the mass fraction of 2-methallyl chloride is 1.3%, the mass fraction of 2-methallyl valerate is 81%, and the mass fraction of 2-methallyl alcohol is 16.6%. Add the organic phase to a three-necked flask, add 335.6g of potassium carbonate solution with a mass fraction of 30%, and hydrolyze at 50°C for 1 hour. After the hydrolysis was completed, the reaction solution was allowed to stand and separate into layers to obtain 44.8 g of an organic phase and 378.5 g of an aqueous phase. Through gas chromatography analysis, the mass fraction of 2-...
Embodiment 3
[0039] In a Hastelloy autoclave, 57g of 2-methallyl chloride, 45.3g of water, 60.4g of sodium propionate and 0.29g of tetrabutylammonium chloride were added, the reaction temperature was controlled at 250°C, and the esterification reaction was carried out for 0.5h. The reaction solution was left to stand and separated to obtain 70.9 g of an organic phase and 92.1 g of an aqueous phase. Through gas chromatography analysis, in the organic phase, the mass fraction of 2-methallyl chloride is 1.4%, the mass fraction of 2-methallyl propionate is 81.7%, and the mass fraction of 2-methallyl alcohol is 16.7%. Add the organic phase to a three-necked flask, add 108.5 g of 35% sodium bicarbonate solution, and hydrolyze at 10°C for 4 hours. After the hydrolysis was completed, the reaction solution was left to stand and separated to obtain 45 g of an organic phase and 134.4 g of an aqueous phase. Through gas chromatography analysis, the mass fraction of 2-methallyl chloride in the organic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com