The method for synthesizing phosphoramidone by hydrogen phosphite diester intermediate
A technology for the synthesis of hydrophosphite diester and phosphoramidone, which is applied in the production of peptides and bulk chemicals, can solve the problems of high polarity of precursors and difficult purification, and achieves improved yield and simplified deprotection. The effect of the steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
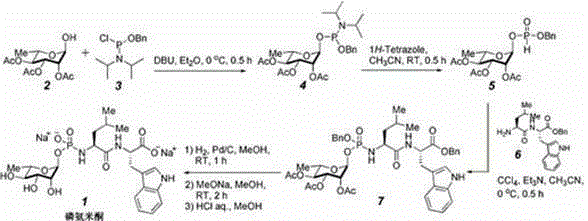
[0014] Embodiment 1: a method for synthesizing phosphoramidone by hydrogen phosphite diester intermediate, comprises the following steps:
[0015] Step 1: O-Benzyl-O-(α-L-2,3,4-triacetyl)-L-rhamnose-1-phosphorous diisopropylamine 4 Synthesis of: 580mg (2.0mmol, 1.0 equivalent) 2,3,4-triacetyl-α-L-rhamnose 2 and 0.51ml (3.4mmol, 1.7 equivalent) of dry DBU were dissolved in 10ml of dry ether, and a solution of 820mg (3.0mmol, 1.5 equivalent) of benzyloxydiisopropylaminophosphorous acid chloride was added dropwise under ice-bath conditions 3 5ml of ether solution. The system gradually had a white precipitate, and the reaction was continued for 30 minutes after the dropwise addition was completed. The reaction solution was concentrated under reduced pressure, and the residue was azeotroped twice with acetonitrile (5 ml) under reduced pressure. The residue was dissolved in ethyl acetate (5 ml), and the insoluble matter was filtered off. The filtrate was concentrated to give a l...
Embodiment 2
[0019] Embodiment 2: a method for synthesizing phosphoramidone by hydrogen phosphite diester intermediate, comprises the following steps:
[0020] Step 1: O-Benzyl-O-(α-2,3,4-triacetyl)-L-rhamnose-1-phosphoramidite 4 Synthesis of: 580mg (2.0mmol, 1.0 equivalent) 2,3,4-triacetyl-α-L-rhamnose 2 and 0.51ml (3.4mmol, 1.7eq) of dry DBU were dissolved in 10ml of dry tetrahydrofuran, and 820mg (4.0mmol, 2.0eq) of benzyloxydiisopropylamidophosphorous acid was added dropwise at -20°C Acid chloride 3 5ml of tetrahydrofuran solution. After the dropwise addition was completed, the reaction was continued for 30 minutes. The reaction solution was concentrated under reduced pressure, and the residue was azeotroped twice with acetonitrile (5 ml) under reduced pressure. The residue was dissolved in ethyl acetate (5 ml), and the insoluble matter was filtered off. The filtrate was concentrated to give a light yellow oily phosphoramidite intermediate 4 The crude product was directly used in...
Embodiment 3
[0024] Embodiment 3: a method for synthesizing phosphoramidone by hydrogen phosphite diester intermediate, comprises the following steps:
[0025] Step 1: O-Benzyl-O-(α-2,3,4-triacetyl)-L-rhamnose-1-phosphoramidite 4 The synthesis of: 1.16g (4.0mmol, 1.0 equivalent) 2,3,4-triacetyl-α-L-rhamnose 2 and 1.2ml (4.0mmol, 2.0eq) of dry DBU were dissolved in 20ml of dry diethyl ether, and 1.64g (8.0mmol, 2.0eq) of benzyloxydiisopropylaminoimide was added dropwise at -20°C Phosphorus oxychloride 3 10ml of diethyl ether solution. After the dropwise addition was completed, the reaction was continued for 1 hour. The reaction solution was concentrated under reduced pressure, and the residue was azeotroped twice with acetonitrile (5 ml) under reduced pressure. The residue was dissolved in ethyl acetate (5 ml), and the insoluble matter was filtered off. The filtrate was concentrated to give a light yellow oily phosphoramidite intermediate 4 The crude product was directly used in the n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com