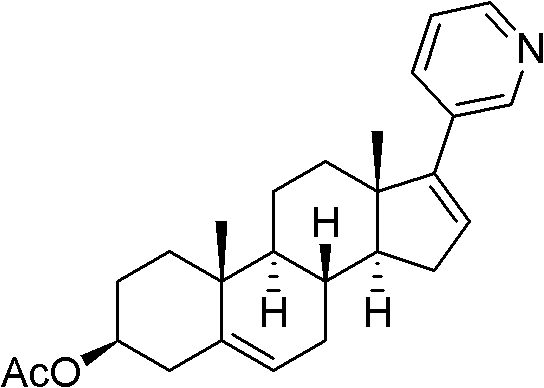
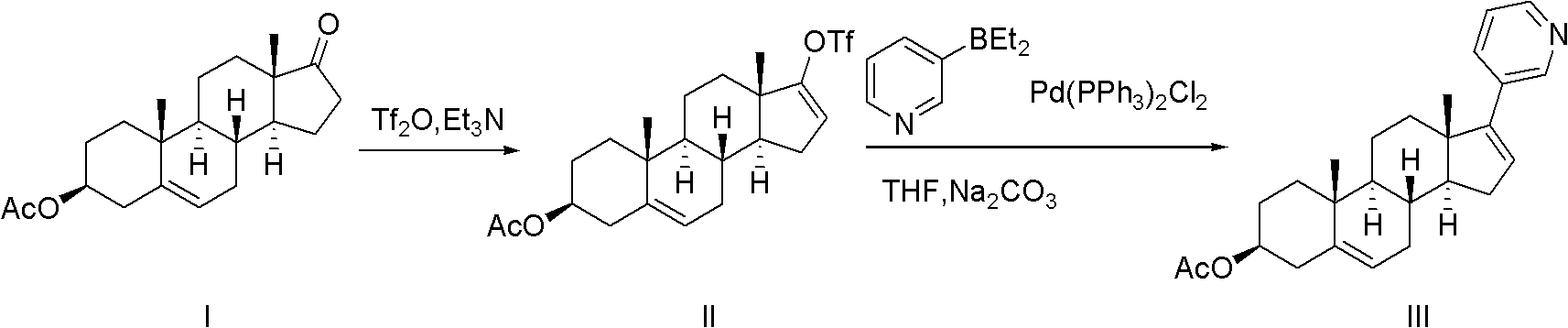
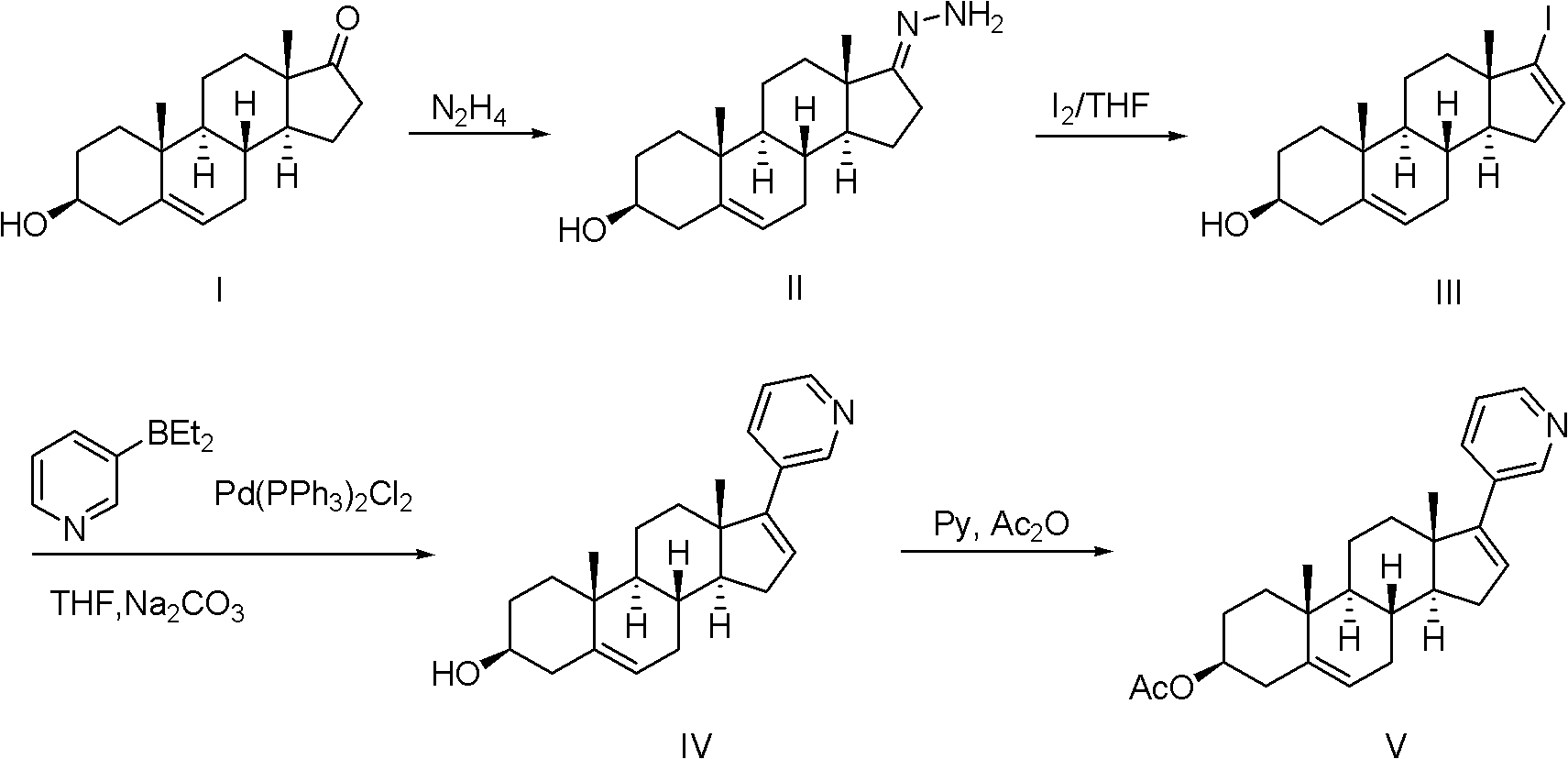
Abiraterone acetate trifluoroacetate, and preparation method and application thereof
A technology of abiraterone acetate and trifluoroacetate, applied in carboxylate preparation, steroids, organic chemistry, etc., can solve the problems of low product purity and yield, unfavorable large-scale production, and many impurities. Achieve the effects of stable quality, simple operation of salt formation and purification, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: Preparation of abiraterone acetate trifluoroacetate
[0034] ① Preparation of Abiraterone Acetate Crude Product
[0035] a) Add 33g of dehydroepiandrosterone acetate into a dry 1L four-necked bottle, vacuumize and protect with argon; add 400ml of dichloromethane, stir to dissolve; control the temperature at 0-30°C, and add trifluoromethanesulfonic anhydride dropwise 23.5ml, the dropwise addition time is 10-20 minutes, after the dropwise addition, the internal temperature is 0-30°C and stirred for 10 minutes; dropwise add 200ml of dichloromethane solution containing triethylamine, the temperature is kept at 0-30°C, the dropwise addition time 35 to 40 minutes; after the dropwise addition, stir at an internal temperature of 0 to 30°C for 1 hour, and perform thin layer chromatography (monitored by TLC); add 250ml of ice water dropwise to stop the reaction, then add 250ml of dichloromethane, separate and collect The dichloromethane layer and the water layer we...
Embodiment 2
[0041] Embodiment 2: be used for purifying abiraterone acetate
[0042] a) Transfer the above-mentioned 28.1g of abiraterone acetate trifluoroacetate to a 500ml single-necked bottle, add 300ml of isopropanol, heat to reflux, cool down to room temperature after dissolving, filter with suction, and wash the filter cake with a small amount of ice isopropanol , drying the filter cake to obtain 21.66g light yellow solid, which is purified abiraterone acetate trifluoroacetate, and the mass yield is 77.1%;
[0043] b) Add 21.66 g of the light yellow solid obtained in 200 ml of dichloromethane, stir to make it substantially dissolved, then add an aqueous solution of sodium carbonate with a mass percent concentration of 20%, control the pH of the aqueous phase>10, then stir at room temperature for 1 hour, Separation, the aqueous layer was extracted twice with 100ml dichloromethane, the dichloromethane layers were combined, dried over anhydrous sodium sulfate, suction filtered and conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com