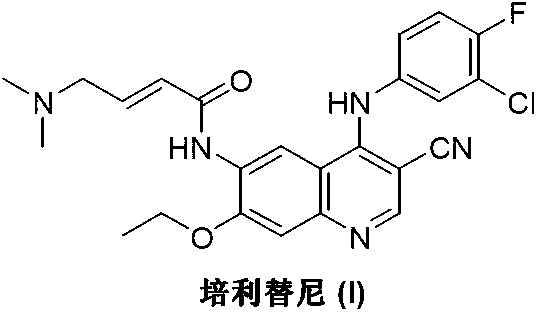
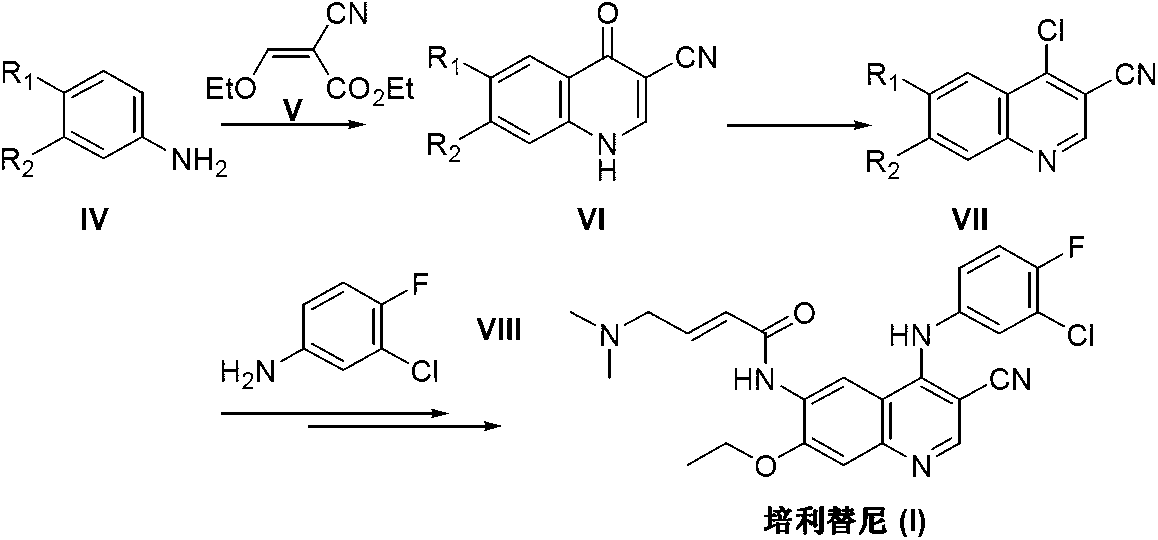
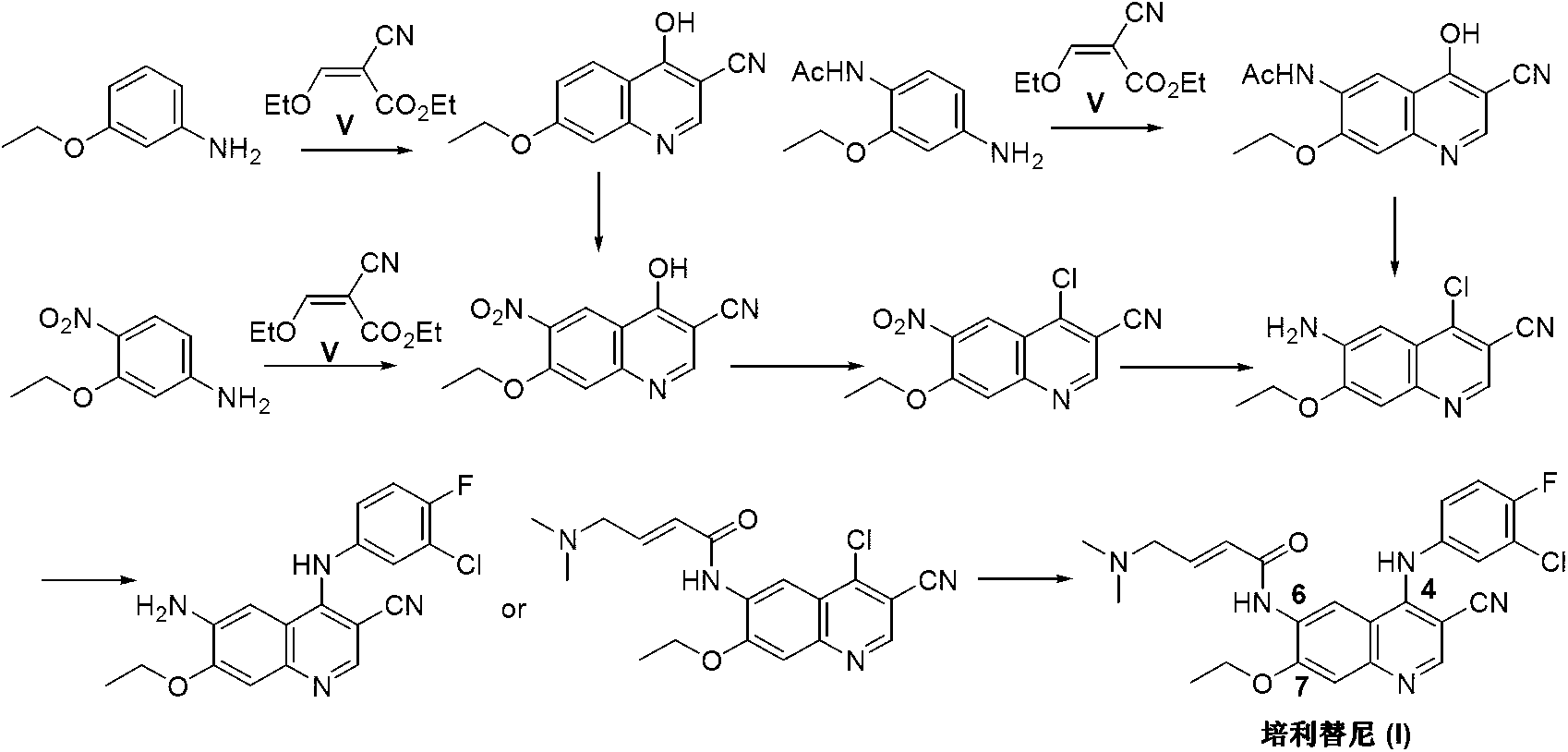
Preparation method of pelitinib
A technology for pelitinib and preparation steps, which is applied in the field of pelitinib preparation, can solve the problems of limiting the industrialization prospect of the process route, high temperature and long-term reflux, etc., and achieves the effects of promoting development, easy availability of raw materials, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 6-[(E)-4-(dimethylamino)-2-butenamido]-7-ethoxy-4-amino-3-quinolinecarbonitrile (II) into the three-neck reaction flask (3.4g, 10mmol), triethylamine (1.5g, 15mmol) and methanol 25mL, warm up to 50-55°C, stir until the system is uniformly dissolved. A methanol solution of 3-chloro-4-fluorobenzaldehyde (III) (1.9 g, 12 mmol) was slowly added dropwise to the reaction liquid, and the drop was completed in about 1 hour. Keep this temperature and continue to react for 3 hours, and TLC detects that the reaction is complete. The temperature was lowered to 0-5°C, and sodium borohydride (1.9 g, 50 mmol) was added in portions, and the addition was completed in about 1 hour. Keep the room temperature to continue the reaction for 2 hours, and TLC detects that the reaction is complete. The reaction was quenched by adding dilute hydrochloric acid. Concentrate under reduced pressure to one-third of the total volume, cool down and crystallize, and recrystallize the crude product...
Embodiment 2
[0030] Under dryness and a nitrogen atmosphere, 3-ethoxy-4-[(E)-4-(dimethylamino)-2-butenamido]aniline (IIa) (2.6g, 10mmol), triethyl orthoformate (IIb) (1.63g, 11mmol) and malononitrile (IIc) (0.80g, 12mmol) and absolute ethanol 25mL, heated to reflux for 3 hours. After cooling and crystallization, the crude product was dissolved in 25 mL of N,N-dimethylformamide, then aluminum trichloride (5.32 g, 40 mmol) was added, heated to 140° C., and kept for 2 hours for reaction. After cooling, the reaction system was poured into ice water, and a solid precipitated out. Filtration, the filtrate was extracted with dichloromethane, concentrated, and the crude product was recrystallized from ethanol to obtain a white solid 6-[(E)-4-(dimethylamino)-2-butenamido]-7-ethoxy-4 -Amino-3-quinolinecarbonitrile (II) 2.9g, yield 85.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com