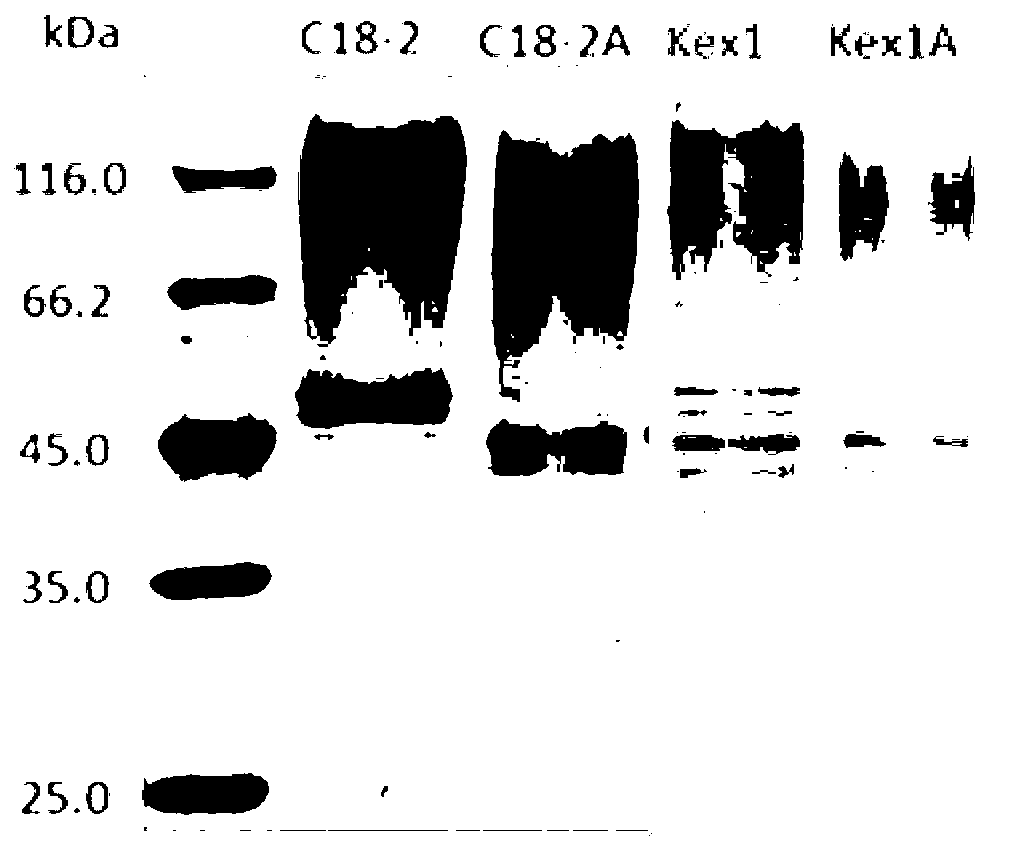
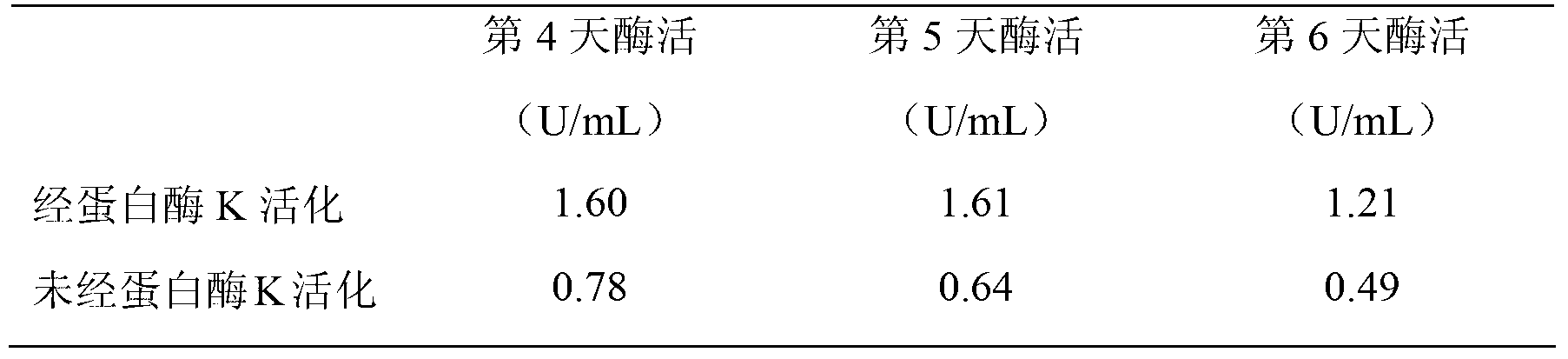
Gene engineering bacteria highly expressing transglutaminase, and applications thereof
A technology of transglutaminase and transglutaminase enzyme, which is applied in the field of genetically engineered bacteria expressing high-efficiency transglutaminase, to reduce production costs and simplify production steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The acquisition of embodiment 1 proTG zymogen
[0038] proTG can be amplified from the previously constructed vector pET22b(+) / proTG (published articles) in our laboratory or artificially synthesized according to the relevant information of Genebank: EU477523.
[0039] Specifically, the recombinant strain construction method is as follows:
[0040] (1) proTG primer design
[0041] The PCR primers P1 and P2 of proTG gene were designed according to pET22b(+) / proTG gene sequence.
[0042] P1: 5'-TTG GGCCGTTCTGGCC GCCAGCGGCGGCGACG-3'(SfiI)
[0043] P2:5'-CGC GGATCC TTACGACCAGCCCTGCTTCACCTC-3' (BamHI)
[0044] (2) Construction of recombinant expression vector pINA1297-proTG
[0045] Using primers P1 and 2, using the pET22b(+) / proTG plasmid as a template, the PCR product of proTG was amplified, and the PCR product was recovered according to the instructions of the gel recovery kit of Fermentas, and the recovered fragment and plasmid pINA1297 were subjected to SfiI sing...
Embodiment 2
[0046] Embodiment 2: The primer design of introducing the cleavage site Lys-Arg of Kex2 protease
[0047] According to the gene sequence of plasmid pINA1297-proTG, PCR mutation primers Kex1 and Kex2 introducing the cleavage site Lys-Arg (the corresponding nucleotide sequence is AAGCGA) were designed.
[0048] Kex1: 5'- CGA GACGCTGCCGACGAGAGGG-3' (the underlined part is the base sequence introduced)
[0049] Kex2: 5'- CTT GGGGGCCCGGAAGAGCG-3' (the underlined part is the base sequence introduced)
Embodiment 3
[0050] Example 3: Amplification of mutant genes
[0051] Using primers Kex1 and Kex2, using the plasmid pINA1297-proTG as a template, the amplification conditions are: 95°C pre-denaturation, 5min, one cycle; 95°C denaturation, 30s, 65°C annealing, 30s, 72°C extension, 6min35s, 34 cycles ; 72°C, 10min, one cycle; 12°C, 10min, one cycle. PCR amplification system: 5×PCR buffer (Mg 2+ Plus) 10 μL, dNTP Mix 4 μL, template 1 μL, upstream and downstream primers 0.1 μL, sterilized double distilled water 35 μL, Prime Star DNA polymerase 0.5 μL. The PCR product was purified and recovered using a gel recovery kit, and the concentration of the recovered product was checked by electrophoresis. The recovered product was stored in a 1.5mL centrifuge tube and stored in a -20°C refrigerator for future use.
[0052] Example 3: Construction of recombinant expression vector pINA1297-proKexTG
[0053] Phosphorylate and ligate the recovered fragments with the Takara Mutation Kit. The specific s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com