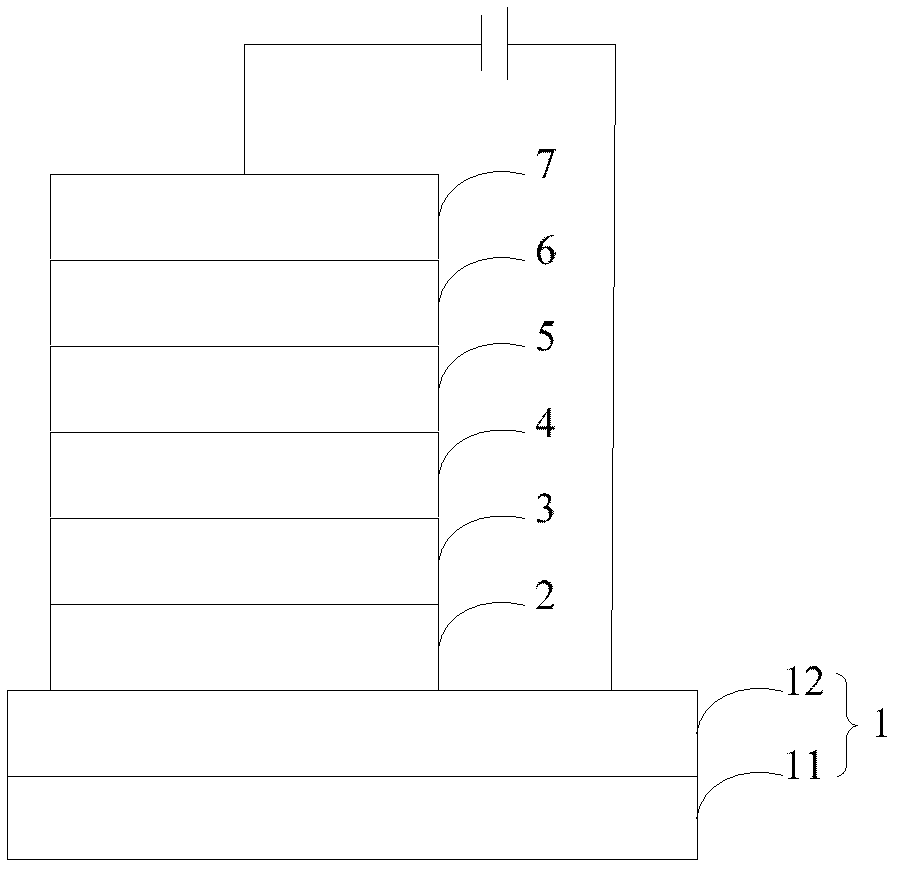
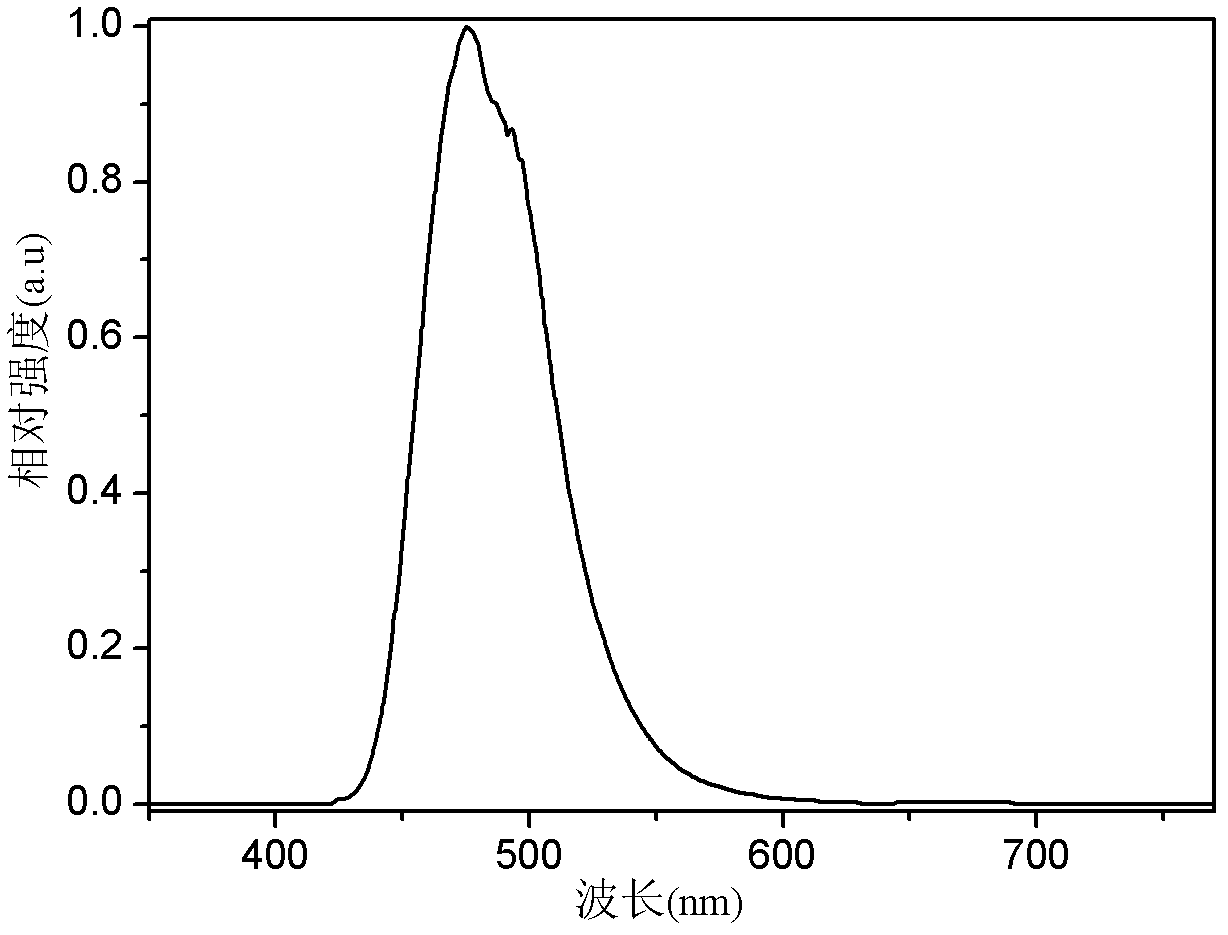
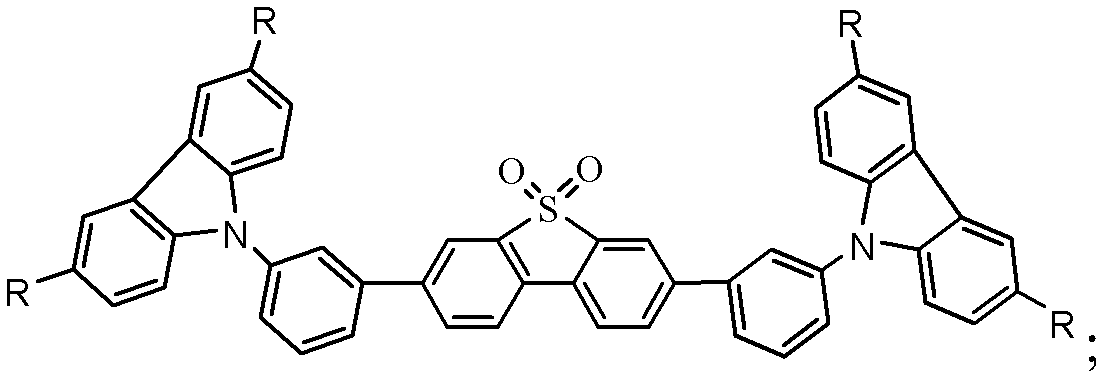
Organic semiconductor material containing dibenzothiophene sulfone, preparation method of organic semiconductor material and organic electroluminescent device
An organic semiconductor, benzothiophene sulfone technology, applied in the field of organic electroluminescent materials, can solve the problems of less electroluminescent blue light, high mobility, lack of high thermal stability, etc., achieve easy control, improved electron transport performance, excellent thermal stability The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] This embodiment discloses a dibenzothiophene sulfone-containing organic semiconductor material with the following structure, namely 2,7-bis(3-(carbazol-9-yl)phenyl)dibenzothiophene sulfone (DCzFSO):
[0029]
[0030] Step 1, the preparation of 2,7-dibromodibenzothiophene sulfone:
[0031]
[0032] Dissolve 4 mmol of dibenzothiophene sulfone in 30 ml of concentrated H 2 SO 4 In, add 8.2mmol NBS at room temperature, stir. After 24 hours, the reaction solution was poured into water, filtered with suction, washed with water and methanol. The remaining solid was recrystallized from chlorobenzene to give 2,7-dibromodibenzothiophene sulfone as a colorless needle solid. Yield: 49%. MS: m / z 374 (M + ).
[0033] Step 2, the preparation of 2,7-bis(3-(carbazol-9-yl)phenyl)dibenzothiophene sulfone (DCzFSO):
[0034]
[0035] Add 3 mmol of 2,7-dibromodibenzothiophene sulfone, 6.3 mmol of 3-(carbazol-9-yl)phenylboronic acid, and 0.27 mmol of tetrakistriphenylphosphine ...
Embodiment 2
[0037] This embodiment discloses a dibenzothiophene sulfone-containing organic semiconductor material with the following structure, that is, 2,7-bis(3-(3,6-di-tert-butylcarbazol-9-yl)phenyl)dibenzothiophene sulfone (Dt-BuCzFSO):
[0038]
[0039] Step 1: same as step 1 of embodiment 1;
[0040] Step 2: Preparation of 2,7-bis(3-(3,6-di-tert-butylcarbazol-9-yl)phenyl)dibenzothiophene sulfone (Dt-BuCzFSO):
[0041]
[0042] 2,7-dibromodibenzothiophene sulfone sulfone 3mmol, 3-(3,6-di-tert-butylcarbazol-9-yl)phenylboronic acid 9.0mmol, tris(dibenzylideneacetone)dipalladium 0.03mmol Add it into the reaction bottle, vacuumize and circulate nitrogen for 3 times, make the reaction system in anaerobic state, under the protection of nitrogen, add 50mL of toluene solution, 2mol / L K 2 CO 3 45ml of aqueous solution, the mixture was heated to 115°C and refluxed for Suzuki coupling reaction for 30h. After the reaction, the reaction solution was poured into saturated ammonium chlori...
Embodiment 3
[0044] This embodiment discloses a dibenzothiophene sulfone-containing organic semiconductor material with the following structure, that is, 2,7-bis(3-(3,6-di-n-hexylcarbazol-9-yl)phenyl)dibenzothiophene sulfone ( DHCzFSO):
[0045]
[0046] Step 1: same as step 1 of embodiment 1;
[0047] Step 2: Preparation of 2,7-bis(3-(3,6-di-n-hexylcarbazol-9-yl)phenyl)dibenzothiophene sulfone (DHCzFSO):
[0048]
[0049] 2,7-dibromodibenzothiophene sulfone sulfone 3mmol, 3-(3,6-di-n-hexylcarbazol-9-yl)phenyl borate 6.4mmol, bis(triphenylphosphine)palladium dichloride 0.003mmol was added to the reaction flask, and after evacuation and nitrogen circulation for 3 times, the reaction system was in an anaerobic state. Under the protection of nitrogen, 55mL of ethylene glycol dimethyl ether solution and 2mol / L Li 2 CO 3 45ml of aqueous solution, the mixture was heated to 100°C and refluxed for Suzuki coupling reaction for 20h. After the reaction, the reaction solution was poured into s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com