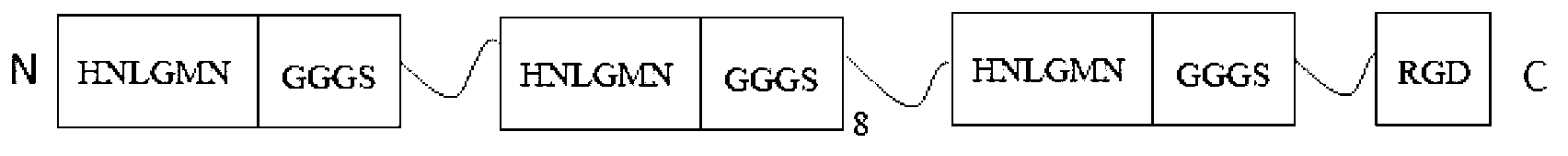Establishment and expression of fusion protein having function of adsorbing heavy metal ions, and application fusion protein in bioremediation
A technology of fusion protein and metal, applied in the field of fusion protein, can solve the problems of insoluble expression, small molecular weight of metallothionein, and many cysteine residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
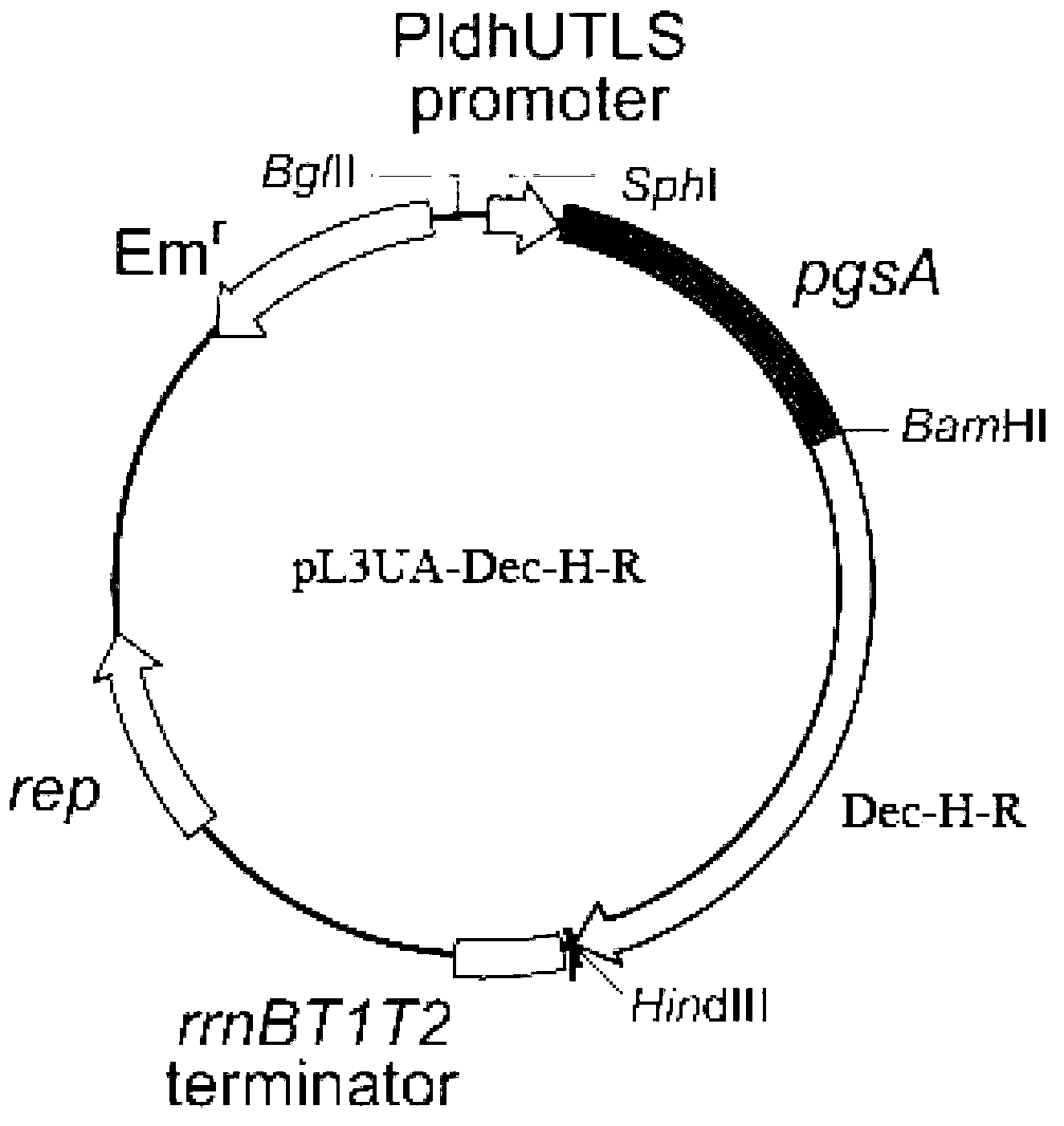
[0062] Embodiment 1: Construction of recombinant food-grade lactic acid bacteria expression vector pL3UA-Dec-H-R
[0063]Escherichia coli (Escherichia coli) NovaBlue (purchased from Novagen, Inc., Madison, Wisconsin) containing the pL3UA plasmid was placed on a Luria-broth (LB) solid culture plate containing erythromycin antibiotics (ingredients: based on the total weight of the medium, 1 % by weight of broth, 0.5% by weight of yeast extract, 1% by weight of sodium chloride, 1.5% by weight of agar powder, and erythromycin at a final concentration of 100 μg / ml) for streak culture. After 14 hours of anaerobic culture at 37°C, pick a single colony from the plate medium and inoculate it into LB liquid medium containing erythromycin antibiotics (ingredients: 1% broth, 0.5% yeast extract, 1% sodium chloride, final erythromycin at a concentration of 100 μg / ml), cultured statically at 37°C until OD 600 = 0.6. The bacteria were collected by centrifugation and the expression plasmid p...
Embodiment 2
[0065] Embodiment 2: the preparation of recombinant lactic acid bacteria and the expression of fusion protein
[0066] Inoculate the recombinant lactic acid bacteria containing the sequenced correct expression plasmid pL3UA-Dec-H-R into the expression medium containing erythromycin (final concentration is 100ng / ml), (ingredients: based on the total weight of the medium, 1 wt% meat peptone, 1 % by weight beef extract, 0.5% by weight of yeast extract, 2% by weight of glucose, 0.5% by weight of sodium acetate, 0.1% by weight of Tween 80, 0.2% by weight of potassium phosphate, 0.2% by weight of ammonium citrate, 0.01% by weight of magnesium sulfate and 0.0005 weight % manganese sulfate, pH = 6.5), 37° C., anaerobic stationary culture for 1 day. After the fermentation, the cells were collected by centrifugation at 12000 rpm, and 0.02 mol / L phosphate buffer (pH=7.0) was added to resuspend the cells at a ratio of 1:10 (v / w). After the cells were disrupted by an ultrasonic disruptor,...
Embodiment 3
[0067] Example 3: Analysis of the Toxic Heavy Metal Ion Binding Ability of the Metal-binding Polypeptide and the Decamer of the Connecting Peptide and the Fusion Protein of the Skin Cell Adhesion Tripeptide
[0068] In the expression medium (ingredients: based on the total weight of the medium, 1% by weight of meat peptone, 1% by weight of beef extract, 0.5% by weight of yeast extract, 2% by weight of glucose, 0.5% by weight of sodium acetate, 0.1% by weight of Tween 80 , 0.2% by weight of potassium phosphate, 0.2% by weight of ammonium citrate, 0.01% by weight of magnesium sulfate and 0.0005% by weight of manganese sulfate, pH=6.5) add different concentrations of cadmium chloride (CdCl 2 , 200μM, 300μM, 400μM, 500μM), lead acetate (PbAc 2 , 0.2mM, 0.3mM, 0.5mM, 1.0mM) or mercury dichloride (HgCl 2 , 15 μM, 50 μM, 150 μM, 450 μM), and then inoculate lactic acid engineered bacteria expressing the fusion protein of the metal-binding polypeptide and the decamer of the connecting...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com