Preparation method of biomimetic mussel adhesive based on synthesis of oxetane derivatives
A technology for oxetane and derivatives is applied in the field of preparation of biomimetic mussel glue, can solve the problems of biomimetic mussel glue biocompatibility, high adhesion strength and difficult to achieve unification, etc., and achieves a simple and efficient synthesis method and biological High compatibility and the effect of breaking through technical bottlenecks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
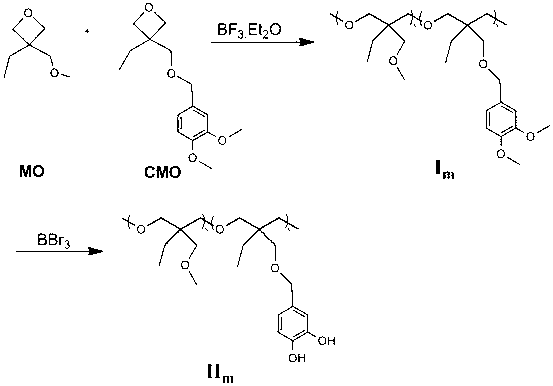
[0034] Add oxetane monomer to the 50ml reaction bottle MO (3.9g 0.03mol), CMO (0.25g 0.93mmol) and dichloromethane (5mL), through argon for 30min, add BF with a syringe 3 ﹒ Et 2 O (19uL), react at 0°C for 6 hours under the protection of argon, add absolute ethanol (2mL) to terminate the reaction, drop the concentrated reaction solution into absolute ethanol (100mL) under stirring, and dissolve the precipitate repeatedly Precipitated three times and dried to obtain the copolymer I m (3.3g) (M n =25KDa M w / M n =1.7).
[0035] Add the prepared copolymer in the 50ml reaction bottle I m , tetrahydrofuran (6mL), boron tribromide (0.7g 2.8mmol), and react under the protection of argon for 10 hours. The concentrated reaction solution was dropped into n-hexane (50mL) under stirring, and the precipitate was dissolved and precipitated three times, and dried to obtain the polymer Ⅱm (2.8g). polymer Ⅱm Dissolve in 5mL of absolute ethanol, add 0.05g of ferric c...
Embodiment 2
[0038]
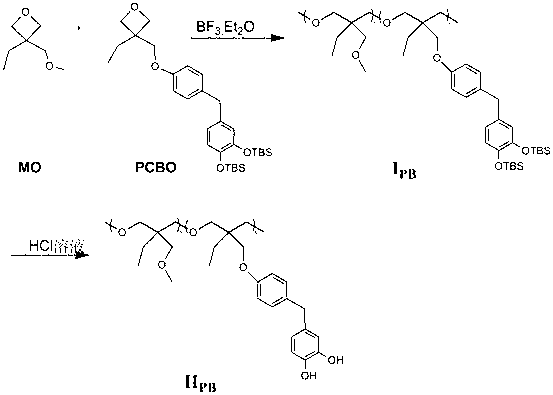
[0039] Add oxetane monomer to the 50ml reaction bottle MO (3.9g 0.03mol), HCBO (4.0g 0.0075mol) and dichloromethane (10mL), through argon for 30min, add BF with a syringe 3 ﹒ Et 2 O (19uL), react at 0°C for 6 hours under the protection of argon, add absolute ethanol (2mL) to terminate the reaction, drop the concentrated reaction solution into absolute ethanol (100mL) under stirring, and dissolve the precipitate repeatedly Precipitated three times and dried to obtain the copolymer I HB (6.3g) (M n =24KDa M w / M n =1.9).
[0040] Add the prepared copolymer in the 50ml reaction bottle I HB , tetrahydrofuran (15mL), concentrated hydrochloric acid (2mL), and reacted for 10 hours under the protection of argon. The concentrated reaction solution was dropped into n-hexane (50mL) under stirring, and the precipitate was dissolved and precipitated three times, and dried to obtain the polymer II HB (5.1g). polymer II HB Dissolve in 8mL of absolute ethanol, a...
Embodiment 3
[0043]
[0044] Add oxetane monomer to the 50ml reaction bottle HO (5.58g 0.03mol), MHCBO (3.92g 0.0075mol) and dichloromethane (10mL), through argon for 30min, add BF with a syringe 3 ﹒ Et 2 O (19uL), react at 0°C for 6 hours under the protection of argon, add absolute ethanol (2mL) to terminate the reaction, drop the concentrated reaction solution into absolute ethanol (100mL) under stirring, and dissolve the precipitate repeatedly Precipitated three times and dried to obtain the copolymer I HBM (7.6g) (M n =20KDa M w / M n =2.0 ).
[0045] Add the prepared copolymer in the 50ml reaction bottle I HBM , tetrahydrofuran (15mL), concentrated hydrochloric acid (2mL), and reacted for 10 hours under the protection of argon. The concentrated reaction solution was dropped into n-hexane (50mL) under stirring, and the precipitate was dissolved and precipitated three times, and dried to obtain the polymer II HBM (5.9g). polymer II HBM Dissolve in 8mL of absolute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com