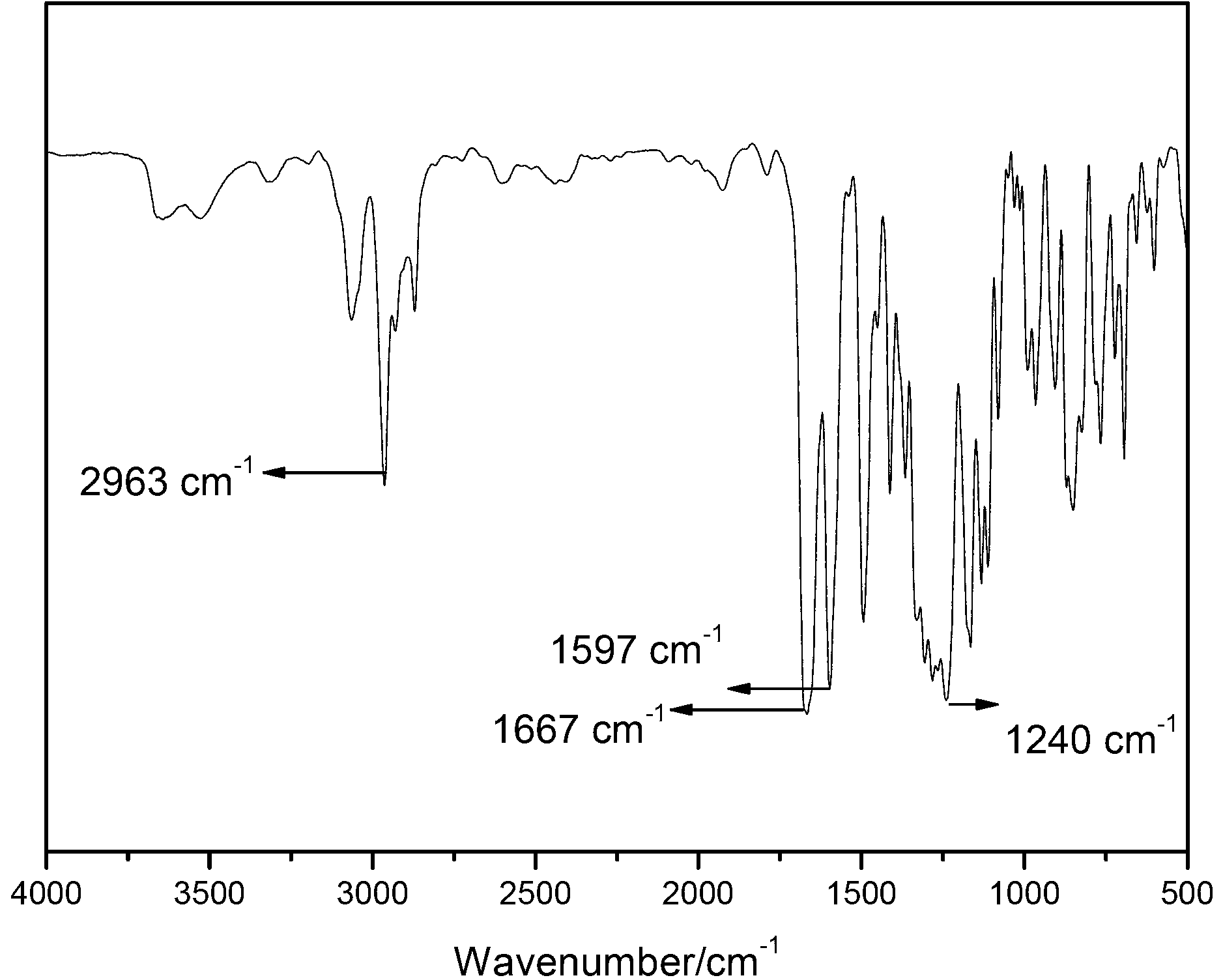
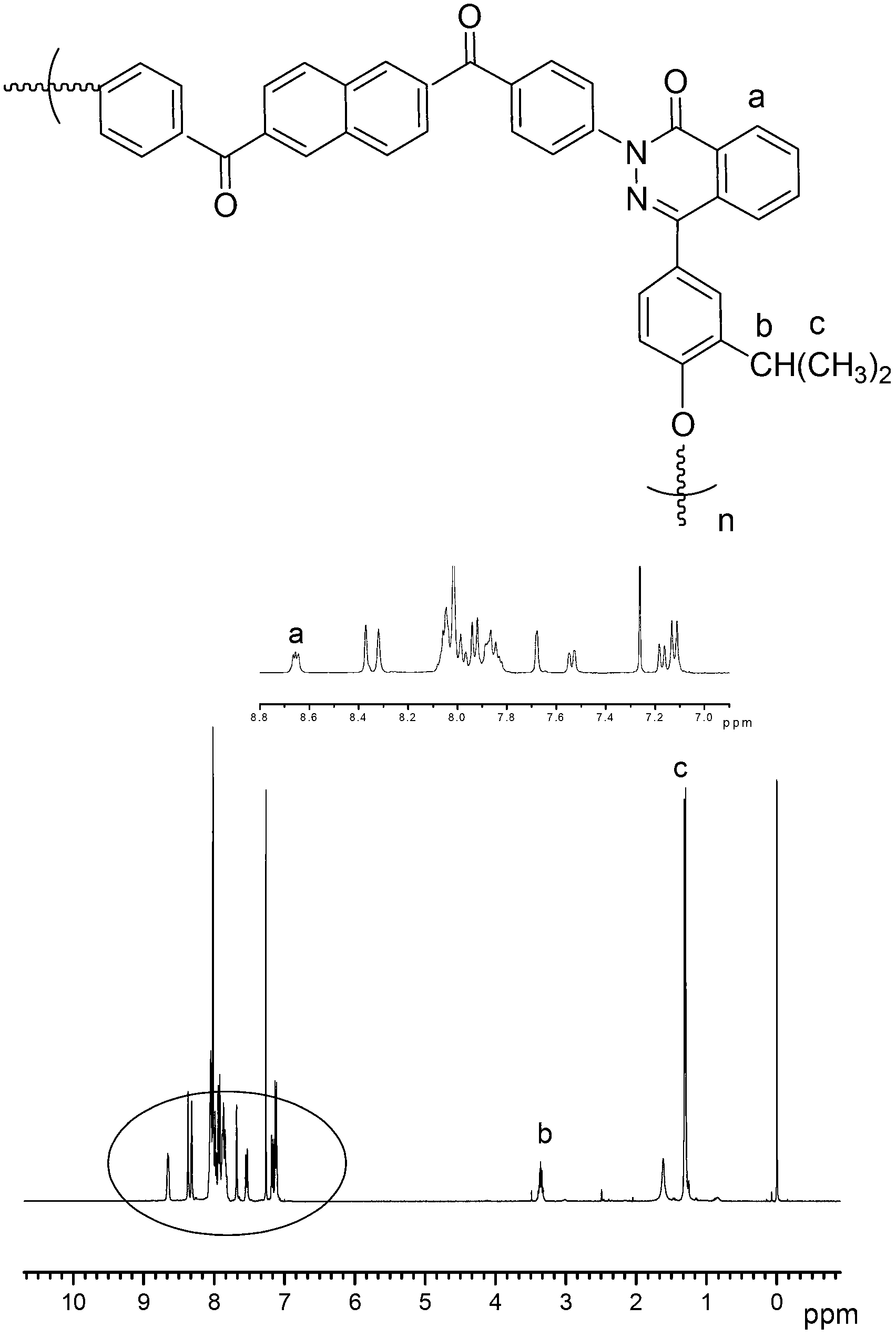
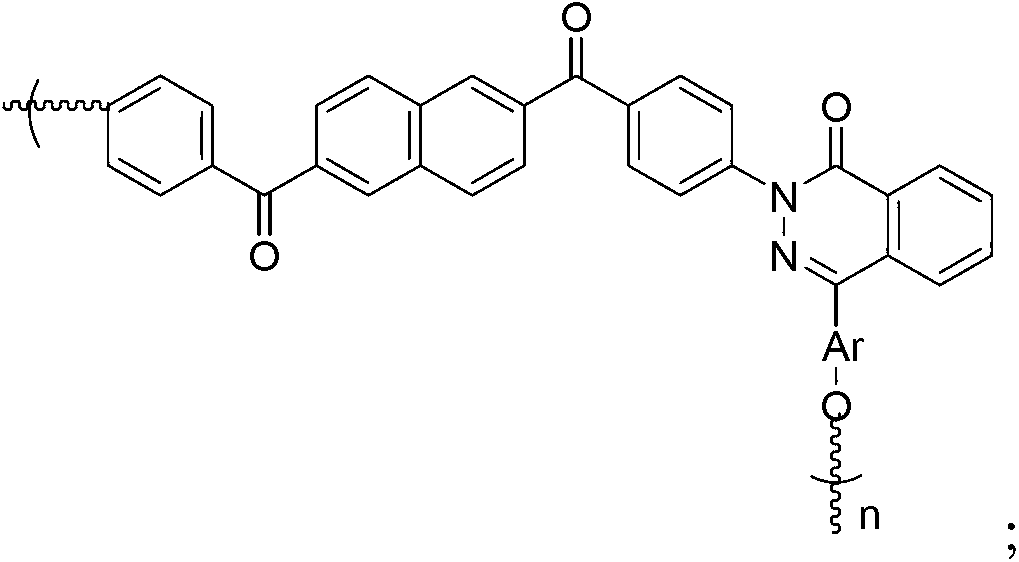
2,6-naphthalic acid based heterocyclic polyaryletherketone and preparation method thereof
A technology of polyaryletherketone and naphthalenedioic acid, which is applied in the field based on 2, can solve problems such as limited application range, difficult processing, and difficult dissolution, and achieves the effects of simple preparation process, low cost, and reduced force between molecular chains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] Step 10, the preparation of phthalazinone monomer: react with phenols of different substituents and phthalic anhydride, the solvent is 1,2-dichloroethane, and the catalyst is AlCl 3 ; The molar ratio of phenol and phthalic anhydride of the different substituents is 1: 1, during preparation, first complete the feeding under the condition of ice bath, then react at room temperature for 1h, then react in 60°C water bath for 3h; glacial hydrochloric acid After settling, let it stand, and obtain the intermediate acid by suction filtration; then, the intermediate acid is subjected to steam distillation, suction filtration, and drying; finally, the dried intermediate acid is dissolved in n-butanol, heated to reflux, and then Add hydrazine hydrate dropwise, the molar ratio of the intermediate acid to hydrazine hydrate is 1:1.5; after the dropwise addition, react for 2 hours, and filter with suction to obtain the phthalazinone monomer;
[0032] The structural formula of the inte...
Embodiment 1
[0049] (1) Preparation of phthalazinone monomer: in a three-necked flask equipped with a mechanical stirring device, an exhaust gas absorption device, and a thermometer, add 2-isopropylphenol 0.05mol (6.8g) and the solvent is 1,2 dichloro Ethane, ice bath. When the temperature drops to about 0°C, add anhydrous AlCl three times at intervals of about 15 minutes. 3 A total of 0.15 mol (20.15 g). anhydrous AlCl 3 After the dissolution was complete, 0.05 mol (7.41 g) of phthalic anhydride was added three times. After dissolving, react at room temperature for 1 h, then react at 60°C for 3 h. The product was poured into 300ml of glacial hydrochloric acid to settle while stirring, and the intermediate acid 2-(3'-isopropyl-4'hydroxybenzoyl)benzoic acid was obtained by suction filtration.
[0050] The obtained intermediate acid was steam distilled until the massive solid became a powder, and no oil phase was distilled out, filtered with suction, and dried. The obtained intermediate...
Embodiment 2
[0062] This part differs from Example 1 in that:
[0063] Described intermediate acid 2-(4'-hydroxybenzoyl) benzoic acid structural formula is as follows:
[0064]
[0065] Ar is
[0066] The structural formula of the 4-(4'-hydroxyphenyl)-2,3-naphthyridine-1-one is as follows:
[0067]
[0068] Ar is
[0069] The polyaryletherketone structural formula is as follows:
[0070]
[0071] Ar is
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com