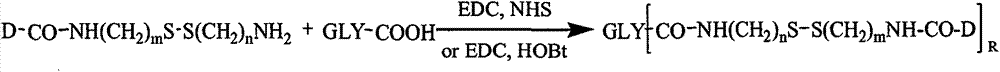Amphiphilic polysaccharide-anti-tumor medicament conjugate capable of releasing medicines specifically at lesion site of living body, as well as preparation method and application of medicinal composition of amphiphilic polysaccharide-anti-tumor medicament conjugate
A technology of anti-tumor drugs and amphiphilic polysaccharides, which is applied in the direction of anti-tumor drugs, drug combinations, medical preparations of non-active ingredients, etc. It can solve the problems of slow shedding of hydrophobic anti-tumor drugs, harsh synthesis conditions, and single mechanism of action. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Example 1: Preparation of hyaluronic acid-cystamine-paclitaxel conjugate
[0113] 0.1 mmol of hyaluronic acid, 1 mmol of cystamine, 0.2 mmol of EDC and 0.2 mmol of NHS were dissolved in formamide, reacted for 24 hours, precipitated with excess acetone, and filtered with suction. Add water to redissolve the precipitate, dialyze with distilled water for 3 days (MWCO=3500), and freeze-dry to obtain the hyaluronic acid intermediate with free one-terminal amino group.
[0114] Dissolve 0.4mmol paclitaxel in pyridine, add 0.4mmol succinic anhydride, and stir at room temperature for 4h. Pyridine was removed by rotary evaporation and dried in vacuo. Add an appropriate amount of double-distilled water, stir for 30 minutes, collect the precipitate by filtration, dissolve the precipitate in an appropriate amount of acetone, slowly add double-distilled water, collect the crystals, and dry in vacuum to obtain a carboxyl-containing 2'-succinyl paclitaxel derivative.
[0115] 0.025m...
Embodiment 2
[0116] Embodiment 2: preparation of chitosan-3,3'-dithiodipropionic acid-paclitaxel conjugate
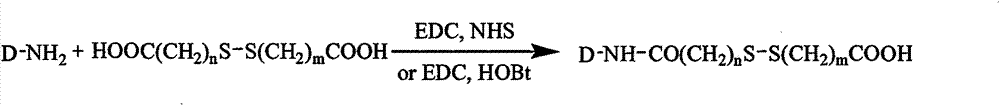
[0117] Dissolve 0.1mmol chitosan in a mixed solvent of water and methanol (v / v=1:1), add 1mmol 3,3'-dithiodipropionic acid, 0.1mmol EDC and 0.1mmol HOBt, react for 8h, remove by rotary evaporation Methanol and distilled water were dialyzed for 3 days (MWCO=3500) to obtain a chitosan intermediate with a free carboxyl group at one end.
[0118] 0.025mmol paclitaxel, 0.1mmol chitosan intermediate, 0.025mmol EDC and a small amount of DMAP were dissolved in a mixed solvent of water and methanol (v / v=1:3), and reacted for 48h. Methanol was removed by rotary evaporation, dialyzed in distilled water for 3 days (MWCO=3500), and freeze-dried to obtain the chitosan-3,3'-dithiodipropionic acid-paclitaxel conjugate carrier.
Embodiment 3
[0119] Embodiment 3: Preparation of chondroitin sulfate-cystamine-gambogic acid conjugate
[0120] 0.1mmol of chondroitin sulfate, 2mmol of cystamine, 0.4mmol of EDC and 0.4mmol of NHS were dissolved in formamide, reacted for 12h, precipitated with excess acetone, and filtered with suction. Add water to redissolve the precipitate, dialyze with distilled water for 3 days (MWCO=3500), and freeze-dry to obtain the chondroitin sulfate intermediate with free one-terminal amino group.
[0121] 0.5mmol gambogic acid, 0.6mmol DCC, and 0.6mmol NHS were dissolved in N,N-dimethylformamide, reacted in ice bath for 30min, and then raised to room temperature for 48h. After the reaction, the precipitate was filtered off, and the gambogic acid-NHS activated ester was purified by silica gel column chromatography using dichloromethane and methanol (v / v=1:1) as the eluent.
[0122] Dissolve 0.4mmol gambogic acid-NHS activated ester and 0.1mmol chondroitin sulfate intermediate in formamide, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com