Fluorescent method of 5-hydroxymethylcytosine based on FRET (Forster Resonance Energy Transfer) principle
A technology for detection of hydroxymethylcytosine and fluorescence, which is applied in the field of fluorescence detection of 5-hydroxymethylcytosine based on the FRET principle, can solve the problems of cumbersome and expensive accuracy of 5-hydroxymethylcytosine, and achieve low cost , Eliminate the effect of fluorescent background
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the synthesis of BODIPY compound with fluorescence:
[0032] Dissolve 5.7g of 2,4-dimethylpyrrole (60mmol) in 120ml of dichloromethane, and drop 5.67g of p-chloromethylbenzenesulfonyl chloride (30mmol) into the stirred 2,4- In the dimethylpyrrole solution, after stirring at room temperature for 12 hours, 20 ml of triethylamine was added to react for 1 hour. After spinning to dryness, passing through a silica gel column, intermediate A of the fluorescent BODIPY compound was obtained with a yield of 27%. 1 H NMR (300MHz, CDCl 3 )δ(ppm):7.51(d,J=6.6Hz,2H),7.26(d,J=6.6Hz,2H),5.97(s,2H),4.65(s,2H),2.54(s,6H) ,1.37(s,6H); 13 C NMR (75MHz, CDCl 3 )δ:155.58,142.96,140.88,138.54,134.98,131.24,129.20,128.33,121.27,45.53,14.52,14.40.HRMS(ESI)calcd for C20H21*BCIF2N2[M+H] + :373.1449;found:373.1452.
[0033] Dissolve 3g of intermediate A (8mmol) of the fluorescent BODIPY compound in 50ml of DMF, add hydroxyphthalimide (2.61g, 16mmol), K 2 CO 3 (3.3g, 24mmol), ...
Embodiment 2
[0035] Embodiment 2: the synthesis of cationic fluorescent polymer:
[0036] Dissolve 325mg of 2,7-dibromo-9,9(6-bromohexyl)fluorene (0.5mmol), 82.9mg of 1,4-benzenediboronic acid (5mmol), 7mg of palladium dichloride in 3ml of 2M potassium carbonate solution and tetrahydrofuran The mixed solution was reacted at 80°C for 24h. The reaction product solution was extracted by adding chloroform and spin-dried. Chloroform and methanol (1:100) were added for recrystallization. The intermediate product C was obtained. The yield was 70%. 1 H NMR (300MHz, CDCl 3 ,ppm):δ7.8(m,5H),7.7-7.6(m,4H),7.5(m,1H),3.3(t,4H),2.1(m,4H),1.7(m,4H), 1.3-1.2(m,8H),0.8(m,4H).Mw:2203g / mol;Mn:1072g / mol;PDI:2.05.
[0037] Dissolve 200 mg of compound C in 5 ml of THF, add 2 ml of trimethylamine solution, stir for 24 h, spin dry, and wash with methanol and ether several times. The gray powder obtained is cationic fluorescent polymer. The yield was 51%. 1 HNMR (300MHz, CD 3 OD,ppm): δ7.9-7.8(m,10H), 3.2(...
Embodiment 3
[0038] Example 3: Based on the principle of FRET, the content of 5-hydroxymethylcytosine in synthetic DNA and genes is detected by fluorescence method.
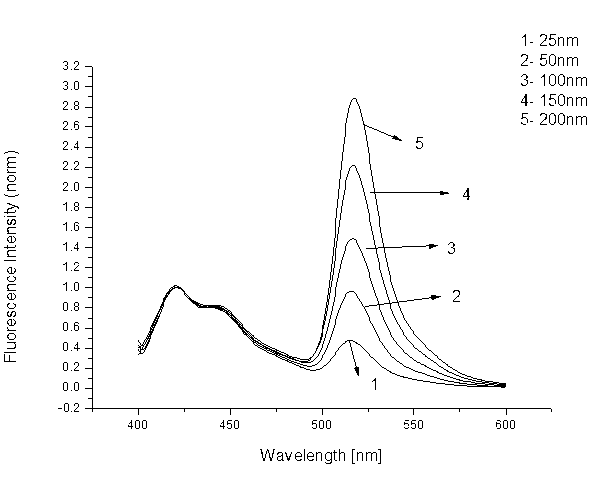
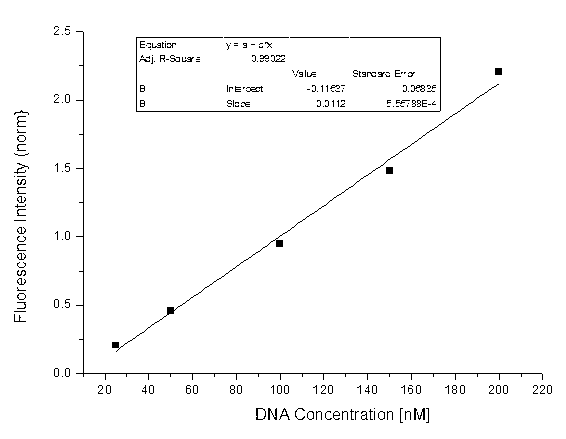
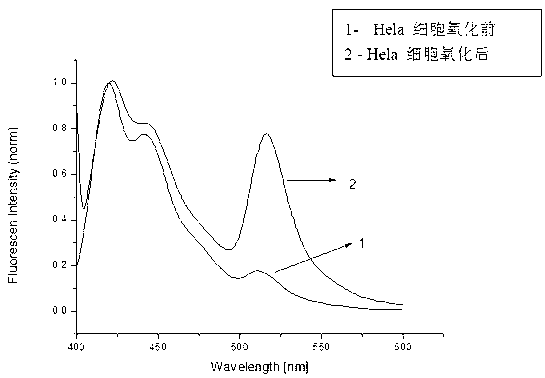
[0039] Take a 1.5ml EP tube, add 75μl of aqueous solution, 10μl of ammonium acetate buffer solution with pH=5-7, 10ul of ammonium amine solution (1M DMSO solution), 4μl of BODIPY fluorescent compound (1mm DMSO solution) solution), and 1 μl of aldehyde-containing cytosine-containing DNA or genomic DNA, reacted at 25-40°C for 12-24h, and extracted three times with chloroform. Then add 1ul of cationic fluorescent polymer (1mm in water). Concussion, centrifugal. Excited with an excitation wavelength of 380 nm. First make the fluorescence map of aldehydecytosine through the synthetic DNA, such as figure 1 As shown, a line graph versus aldehyde group content, such as figure 2 shown; and then quantify the content of hydroxymethylcytosine in the gene by detecting the fluorescence content of the oxidized Hela cell gene, such as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com