Protection-sleeve-carrying paclitaxel drug balloon and preparation method thereof
A technology for protecting sleeves and paclitaxel, which is applied in the fields of drug devices, medical science, and other medical devices, can solve problems such as hidden dangers in safety, toxicity of paclitaxel, and few applications, so as to reduce medical costs, facilitate industrial production, The effect of inhibiting vascular restenosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
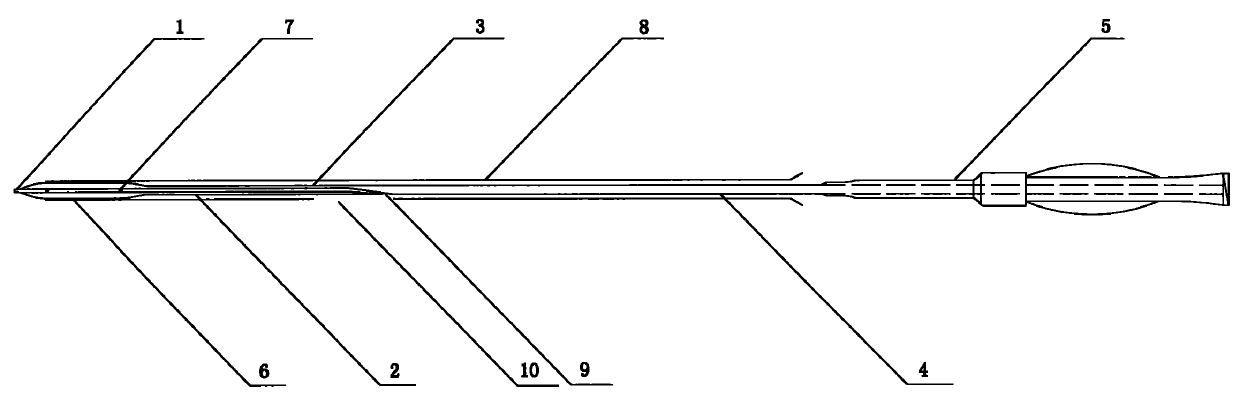
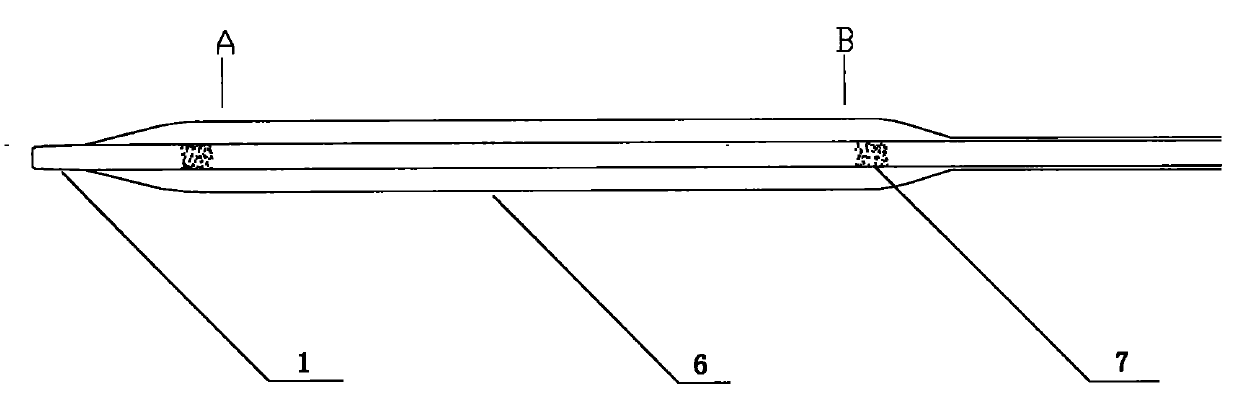
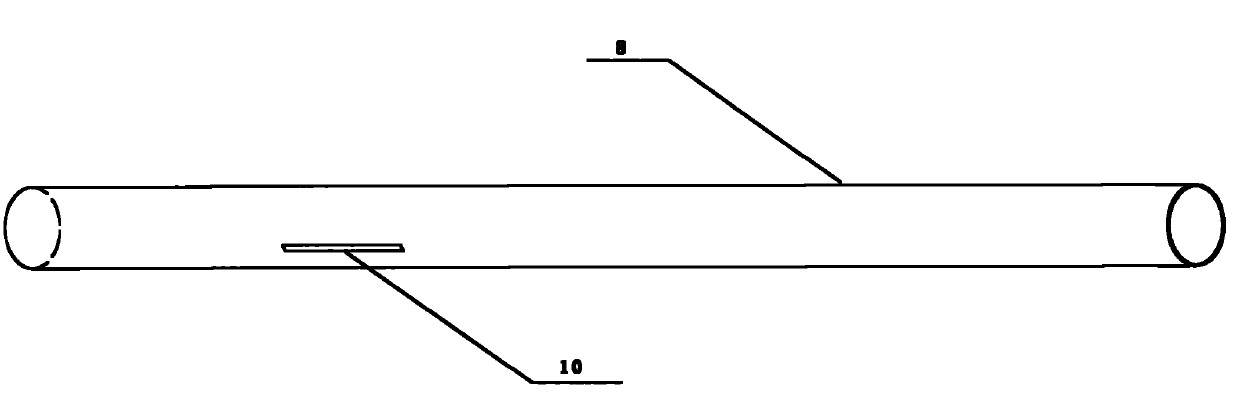
[0046] Such as figure 1 As shown, the balloon of the drug balloon provided in this implementation is coated with a degradable drug coating containing paclitaxel, and a protective sleeve 8 is arranged on the outside of the drug coating, and the protective sleeve 8 covers from the working section of the balloon to the Proximal push rod, the length of the protective sleeve is 130 mm to 138 mm, the inner diameter is 1.3 mm to 1.4 mm, the wall thickness is 0.20 mm to 0.25 mm, and the guide wire opening 10 is arranged longitudinally in the middle, and the size is 0.40 mm (width) × 400 mm (length) .
[0047] The drug balloon comprises a head end 1, a distal push rod 2, a transition section 3, a proximal push rod 4 and a HUB sheath handle 5, the distal push rod 2 includes a balloon 6, and the transition section 3 contains guide wire outlet 9. The push rod 4 at the proximal end of the drug balloon is made of 304L stainless steel tubing, and the push rod 2 at the far end is made of ny...
Embodiment 2
[0068] The difference between this embodiment and the embodiment 1 is that the drug coating includes a solution of paclitaxel and a contrast agent in a ratio of 1 g / 20-35 ml by weight, and the contrast agent is iodobenzene hexitol.
[0069] The preparation method of the paclitaxel drug balloon, its production steps are as follows:
[0070] (1) The nylon tubing is formed into a balloon body, the length of which is 18mm;
[0071] (2) cleaning the balloon body;
[0072] (3) Coating solution configuration and balloon loading drug:
[0073] ①A solution in which paclitaxel and iodobenzene are mixed in a ratio of 1g / 20ml by weight;
[0074] ② Coat the above-mentioned coating solution on the surface of the balloon body, solidify simultaneously with high-purity nitrogen, and keep the pressure at 1.0Psi until the drug loading reaches 320μg / cm 2 ;
[0075] (4) Place the above-mentioned coated balloon body in a vacuum drying oven, and dry it for 12 hours at a vacuum degree of -0.2Mpa ...
Embodiment 3
[0082] The difference between this embodiment and Example 1 is that the drug coating includes paclitaxel, lactide, glycolide copolymer PLGA and 70 parts of acetone in a ratio of 1 part by weight: 1.3 parts ; The preparation method of the paclitaxel drug balloon, its production steps are as follows:
[0083] (1) The nylon tubing is formed into a balloon body, the length of which is 23mm;
[0084] (2) cleaning the balloon body;
[0085] (3) Coating solution configuration and balloon loading drug:
[0086] ① Weigh paclitaxel and PLGA according to the ratio of 1 part: 1.3 parts for preparation;
[0087] ②Add 1.3 parts of PLGA to 70 parts of acetone, and shake it with a vibrating incubator at room temperature until it dissolves and disperses evenly, then add 1 part of paclitaxel to prepare a coating solution;
[0088] ③ Coat the above-mentioned coating solution on the surface of the balloon body, solidify simultaneously with high-purity nitrogen, and keep the pressure at 1.2Psi ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com