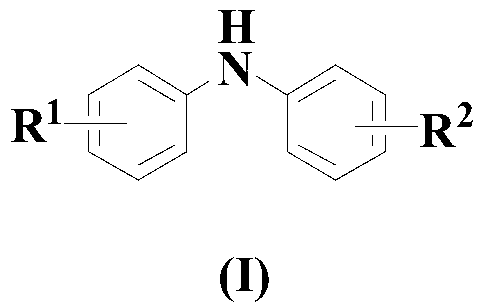
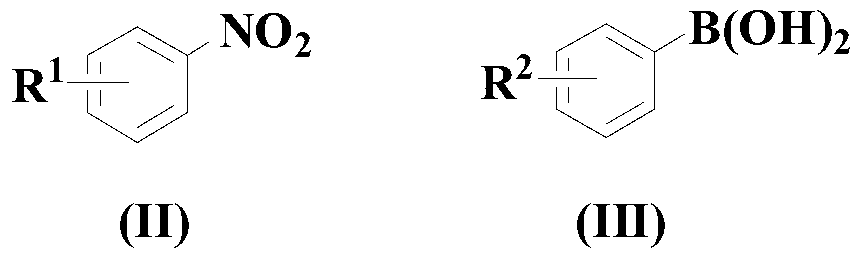
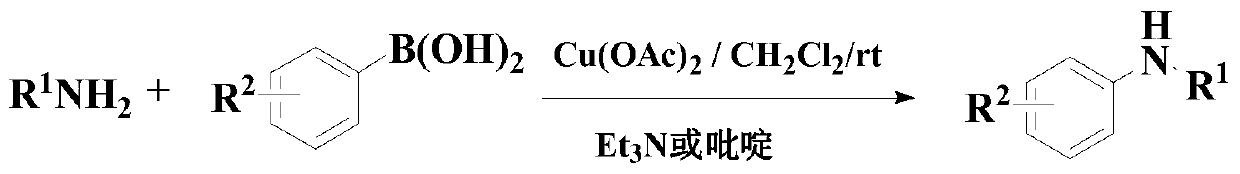Synthesis method of diaryl aniline compound
A technology of diarylaniline and synthesis method, which is applied to the synthesis of diarylaniline compounds and the synthesis field of amine compounds, can solve the problems of limited substrate expansion, narrow substrate range, low utilization efficiency and the like, and achieves good industrialization Prospect, effect of high product yield and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Embodiment 1: the synthesis of diphenylamine
[0078]
[0079] In a dry and clean flask, add 50ml of solvent 1,4-dioxane, and then add the above formula (II) compound, formula (III) compound, Cu(acac) 2 and potassium tert-butoxide so that the molar ratio is 1:2:0.05:2, wherein the compound of formula (II) is 10mmol, nitrogen replacement is performed three times, and then the reaction system is stirred at 80°C under the protection of continuous feeding of nitrogen React for 14 hours.
[0080] After the reaction is finished, filter, and the filtrate is used to remove the solvent by a rotary evaporator, and the residue is purified by 200-300 mesh silica gel column chromatography to obtain the target product as a solid, with a yield of 98.2% and a purity of 98.9% (HPLC) .
[0081] Melting point: 52-53°C;
[0082] NMR: 1 H NMR (CDCl 3 ,500MHz):δ7.23-7.26(m,4H),7.04-7.09(m,4H),6.93(m,2H),5.69(s,1H);
[0083] 13 C NMR (CDCl 3 , 125MHz) δ143.2(2C), 129.4(4C), 121.0(4...
Embodiment 2
[0084] Example 2: Synthesis of phenyl m-tolyl-amine
[0085]
[0086] In a dry and clean flask, add 50ml of solvent 1,4-dioxane, and then add the above formula (II) compound, formula (III) compound, Cu(acac) 2 and potassium tert-butoxide, so that the molar ratio is 1:3:0.1:3, wherein the compound of formula (II) is 10mmol, nitrogen replacement is three times, and then under the protection of continuously feeding nitrogen, the reaction system is stirred at 90°C React for 13 hours.
[0087] After the reaction, filter, and use a rotary evaporator to remove the solvent from the filtrate, and purify the residue through 300-400 mesh silica gel column chromatography to obtain the target product with a yield of 95.3% and a purity of 98.2% (HPLC).
[0088] NMR: 1 H NMR (CDCl 3 ,500MHz):δ7.27-7.24(m,2H),7.15(t,J=7.9Hz,1H),7.06(d,J=7.7Hz,2H),6.93-6.88(m,3H),6.75( d,J=7.5Hz,1H),5.73(s,1H),2.31(s,3H);
[0089] 13 C NMR (CDCl 3 , 125MHz) δ143.2, 143.1, 139.2, 129.3 (2C), 129.1, 12...
Embodiment 3
[0090] Example 3: Synthesis of phenyl-p-methoxyphenyl-amine
[0091]
[0092] In a dry and clean flask, add 50ml of solvent 1,4-dioxane, and then add the above formula (II) compound, formula (III) compound, Cu(acac) 2 and potassium tert-butoxide, so that the molar ratio is 1:4:0.15:4, wherein the compound of formula (II) is 10mmol, and the nitrogen is replaced three times, and then the reaction system is stirred at 100°C under the protection of continuously feeding nitrogen React for 12 hours.
[0093] After the reaction was completed, filter, and use a rotary evaporator to remove the solvent from the filtrate, and purify the residue through 400-500 mesh silica gel column chromatography to obtain the target product with a yield of 96.4% and a purity of 99.1% (HPLC).
[0094] NMR: 1 H NMR (CDCl 3 ,500MHz):δ7.21-7.27(m,2H),7.07-7.11(m,2H),6.95-6.84(m,5H),5.52(s,1H),3.83(s,3H);
[0095] 13 C NMR (CDCl 3, 125MHz) δ144.0, 140.3, 130.9, 129.9 (2C), 129.3 (2C), 120.3 (2C), 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com