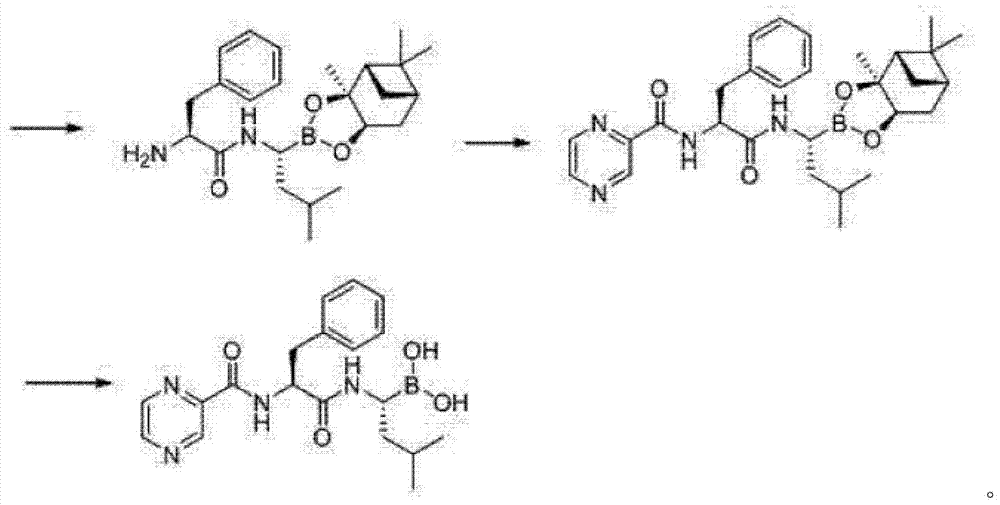
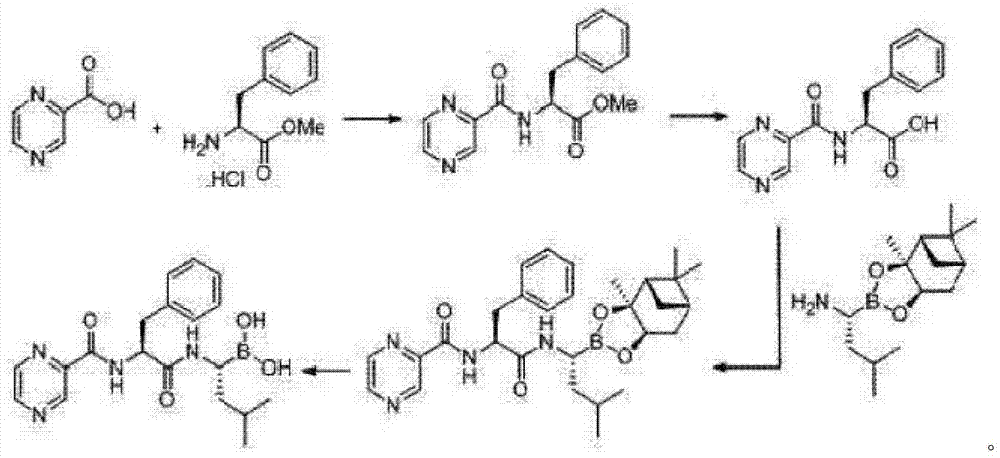
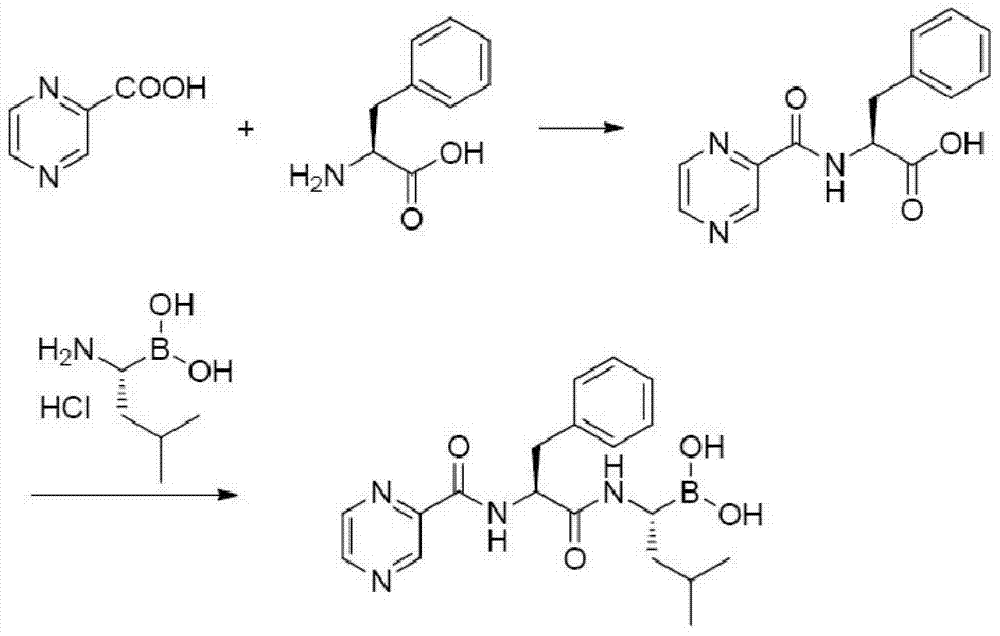
Synthetic method of 26S protease inhibitors
A technology of solvent and condensing agent, applied in the field of medicine, to achieve the effect of reducing production cost, avoiding side reactions and reducing product loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of embodiment 1 (R)-1-amino-3-methylbutane-1-boron hydrochloride
[0048]
[0049]Add (R)-1-amino-3-methylbutane-1-boronic acid pinacol ester hydrochloride (2.5kg, 10mol) into a reaction kettle filled with 25L tetrahydrofuran, and stir to completely dissolve the reaction raw materials. Cool down to 0-5°C, add 2-methylpropaneboronic acid (2.02kg, 20mol) in batches under stirring, maintain the reaction temperature at 0-5°C, monitor the end point of the reaction by TLC, and the reaction ends after 5-8 hours. The reaction solution was neutralized with saturated sodium bicarbonate to pH 7-8. Add dichloromethane for extraction twice, 10 L each time, combine the organic layers, wash the organic layer thoroughly with 20 L of water, add 1 mol / L hydrochloric acid dropwise under stirring at room temperature until no solid precipitates out. After the precipitated solid was washed twice with water, it was vacuum-dried to obtain 1.58 kg of white solid, namely (R)-...
Embodiment 2
[0052] The preparation of embodiment 2 (R)-1-amino-3-methylbutane-1-boron hydrochloride
[0053] Step (1): Preparation of N-(tert-butylsulfinyl)-3-methyl-1-butylimine
[0054]
[0055] Put 3-methylbutyraldehyde (860 g, 10 mol) and tert-butylsulfinamide (1.21 kg, 10 mol) into a reaction kettle filled with 25 L of dichloromethane, stir to make the raw materials completely dissolve, and then add tetrabutyl titanate Ester (341g, 1mol) was refluxed for 8 hours. After the reaction is completed, wash with 10% acetic acid solution (10L), 10% sodium bicarbonate solution (10L) and saturated saline (20L) successively, and concentrate to obtain 1.8kg of colorless oil, which is detected by HPLC (internal standard control ), and its main component was determined to be N-(tert-butylsulfinyl)-3-methyl-1-butylimine with a purity of 95% and a yield of 90.5%.
[0056] Step (2): Preparation of (1R)-(S)-pinanediol-1-(tert-butylsulfinamido)-3-methylbutane-1-boronate
[0057]
[0058] The N...
Embodiment 3
[0067] Example 3 Preparation of N-(pyrazine-2-formyl)-L-phenylalanine
[0068]
[0069] Add pyrazine-2-carboxylic acid (1.24kg, 10mol) into a reaction kettle with 20L of dichloromethane, add O-benzotriazole-N,N,N',N'-tetramethylurea tetrafluoro Borate ester (3.2kg, 10mol) was used as a catalyst, and stirred until the raw material was completely dissolved. Cool down to 0-5°C, dissolve L-phenylalanine (1.65kg, 10mol) and N,N-diisopropylethylamine (1.74L, 10mol) in 5L of dichloromethane, and add dropwise to the reaction In the still, control the dropwise addition within 3 to 5 hours. After the dropwise addition was completed, the reaction mixture was kept at 0-5° C. and stirred for 2 hours, then the temperature was naturally raised to room temperature and stirred for 2 hours, and then the reaction was stopped. Use 1mol / L hydrochloric acid (10L), deionized water (10L), saturated NaHCO 3 (10L) and saturated brine (20L), the organic phase was dried, filtered, concentrated, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com